iodine
0.10
trace elements
under 0.10
Source: DiSpezio, M., et al. 1996. Science insights: Exploring living things. (Teacher’s Edition) Menlo Park, CA: Addison-Wesley Publishing Company, p. 57.`
Master 1.3
Copyright © 2000 by BSCS and Videodiscovery, Inc. Permission granted. Updated 2012.
Questions for Case Studies
1. What happened? Where did it happen? When did it happen?
2. What chemical was involved?
3. What was the route of exposure?
4. What were the symptoms of toxicity?
5. How could a person have prevented his or her exposure to the chemical?
6. Have any changes occurred since the incident? Describe them.
Master 1.4
Copyright © 2000 by BSCS and Videodiscovery, Inc. Permission granted. Updated 2012.
Case Studies of Routes of Exposure
CASE STUDY #1
Dr. Karen Wetterhahn, a Dartmouth College scientist and leading researcher on the impact of toxic metals on living organisms, died in June of 1997 of a lethal dose of dimethylmercury (die-METH-ul-MER-kyoo-ree). She was studying how mercury prevents cells from repairing themselves, a characteristic commonly seen in cancer cells.
Ten months earlier, Dr. Wetterhahn had an accident in her laboratory. A drop of the toxic compound spilled and seeped through her latex gloves. Dr. Wetterhahn followed all the known safety procedures when working with a toxic chemical: She used a hood to protect her from fumes and wore a face shield and gloves. Tests in an independent laboratory after the accident, however, showed that dimethlymercury can pass through latex gloves in 15 seconds or less, usually without damage to the gloves. It came as a surprise to the chemistry department at Dartmouth and to the doctor’s research colleagues that the gloves she was wearing at the time of the spill were not sufficient protection. Since the accident, researchers working with dimethylmercury are urged to wear neoprene gloves with long cuffs or two pairs of gloves, one of them laminated and one of them heavy duty.
Dimethylmercury is a thick, colorless liquid with a sweet odor. It is not the same kind of mercury found in thermometers. Instead, it looks like water but is three times as dense. It is attracted to the oil in human skin and is readily absorbed by the body. It can cause permanent nervous system damage or death. Dr.
Wetterhahn sought medical attention when she felt numbness in her fingers, was unsteady while walking, had difficulty speaking, had blurred vision, and had problems hearing. Within three weeks of diagnosis of mercury poisoning, she went into a coma that lasted until her death four months later.
Poisoning from dimethylmercury is a rare event. According to Dartmouth officials, only one other researcher has died of dimethylmercury poisoning in this century: a Czechoslovakian scientist in 1971.
Before that, two people died from exposure to the chemical when it was first made in the laboratory in the mid-1800s.
Sources: Associated Press. 1997, March 28. Hanover, NH; Associated Press. 1997, June 11. Hanover, NH; Haworth, K. 1997, April 1. Mercury poisoning from lab work puts Dartmouth chemist in hospital. The Chronicle of Higher Education; Holden, C.
(Ed.). 1997, June 20. Random samples: Death from lab poisoning. Science 276, 1797.
Master 1.5a (page 1 of 6)
Copyright © 2000 by BSCS and Videodiscovery, Inc. Permission granted. Updated 2012.
CASE STUDY #2
Early in the morning of December 3, 1984, a young autorickshaw (taxi) driver finally finished his day of work in Bhopal (boh-PAWL), India. As he drove home to his family, he began to notice that the air felt hot even though it was a winter’s night. There was smoke in the air that made his eyes burn.
He arrived at his house and went in, calling to his wife and two children.
“What is going on?” he said.
“We don’t know,” replied his wife, coughing. “But, we have noticed the smell, too. It is making us feel very sick.”
Just then, the young man noticed thick smoke coming in under the door. Outside he heard screaming and shouting. Suddenly, there was a knock on the door. The next-door neighbor shouted through the door, “Run, run away from here. Gas has leaked from the Union Carbide plant and people are collapsing in the streets!”
The man and his wife looked at each other, frightened. “Why was there no siren or warning?” they asked each other.
It was then that the children started crying. They coughed as if they were choking and then vomited, over and over again. The young man covered his mouth with a wet muffler and picked up his children.
He and his wife got into the taxi to begin to drive away from the cloud of gas.
Everywhere in the streets were people running, vomiting, wearing only pajamas. Many people tried stopping the taxi and begged for space, but there was no room for more people. The gas cloud was so thick that the streetlights looked like dim candles burning. Corpses lay in the streets, some bloated to twice their usual size. Police vans were roaming the city, blaring through their loudspeakers,
“Evacuation!” The young man drove on, leaving the city and the terror behind.
The next day, news came that the pesticide plant in Bhopal had leaked the chemical methylisocyanate (METH-ul-IE-soh-SIE-uh-nate). The workers and people living around the plant were exposed to a deadly gas. By the end of the week, at least 6,000 people were dead. When the young man and his family returned to the city, it was like a graveyard.
During the next two months, the man and his family suffered, too. Unable to find medical care, one of the children died a slow, painful death. The man’s wife was no longer able to do her chores because she felt so weak and her lungs always burned. The man worked from dawn to dusk to bring in money for the family and to take care of his wife and child.
Today, thousands of people in Bhopal, India, are still seriously ill. Many people who were exposed to the cloud of gas continue to suffer from diseases related to their exposure to the chemical. They have problems with their lungs, less control over their limb movements, nervous system diseases, and damaged immune systems.
(continued)
Master 1.5b (page 2 of 6)
Copyright © 2000 by BSCS and Videodiscovery, Inc. Permission granted. Updated 2012.
When an investigation into the disaster in Bhopal was completed, Union Carbide was accused of having poor safety and maintenance standards. Defects in the methylisocyanate unit were found in various places: the gauges measuring temperature and pressure were unreliable and ignored; the refrigeration unit had been shut off; the gas scrubber, designed to neutralize any escaping chemical, had been shut off for maintenance; the flare tower, designed to burn off any escaping chemical, was undersized and turned off; the water curtain, designed to neutralize any remaining gas, was too short to reach the tower where the gas was billowing; the alarm on the storage tank did not signal the increase in temperature; and there were inadequate storage tanks for excess chemical. Officials from Union Carbide believed that the leak was the result of sabotage by a disgruntled employee, but the company has never brought charges against anyone.
In February 1989, the Supreme Court of India ordered Union Carbide Corporation (United States) and Union Carbide India Limited, the owner and operator of the chemical plant, to pay $470 million in settlement of all claims arising out of the chemical leak. In addition, the companies were ordered to build a hospital in Bhopal and set up a charitable trust in England to support it. In 1994, Union Carbide Corporation completed the sale of its interest in Union Carbide India Limited to a company in Calcutta.
Part of the proceeds from the sale went to the hospital and clinics in Bhopal.
Source: Testimony of Bhopal Gas Victims. Retrieved August 9, 2000, from http://www.bhopal.org/testimony/jewan_shinde.html.
Master 1.5c (page 3 of 6)
Copyright © 2000 by BSCS and Videodiscovery, Inc. Permission granted. Updated 2012.
CASE STUDY #3
A two-year-old girl named Jane ended up in the emergency room after her mother began to worry about her illness. Jane complained of abdominal pain, she had lost her appetite, she was vomiting, she was constipated and, lately, she seemed very tired.
The doctor in the emergency room asked Jane’s mother where the family lived. He recognized the area as an inner-city neighborhood of old homes, many in poor repair, with peeling and chipping paint. He asked that a blood sample be taken and tested for the level of lead in it. He discovered that Jane’s blood lead level was 100 micrograms of lead per deciliter (1 deciliter = 100 mL) of blood. Because any amount of lead above 10 micrograms per deciliter of blood is dangerous, Jane was at risk of major seizures and cardiac arrest. The doctor told Jane’s mother that Jane was suffering from lead poisoning, but that she would feel better within a short time if she began drug therapy immediately. The drug was easy for Jane to take by mouth, and she could continue her treatment at home.
The cause of Jane’s illness was the lead in the dust, paint, or soil around her home. The doctor told Jane’s mother that the long-term effects of lead in a child can be severe. They include learning disabilities, decreased growth, hyperactivity, impaired hearing, and even brain damage. If caught early, however, these effects can be limited by medical treatment and by reducing exposure to lead.
Jane’s mother wondered why she and her husband were not sick. The doctor explained it this way.
Children swallow lead or breathe lead-contaminated dust when they play in dust or dirt and then put their fingers or toys in their mouths. Often, children eat without washing their hands first. They tend to chew on window ledges and cribs and get lead paint in their mouths. All of these behaviors would be unusual for an adult, so adults are not at as high a risk of exposure. In addition, adults’ organ systems are not affected by lead the way children’s organs are.
When Jane’s mother asked the doctor what she should do to minimize the family’s exposure to lead, the doctor had several suggestions. First, test children living in older homes once a year for high lead levels in their blood. Second, keep areas where children play as clean as possible. Wash things that have fallen on the floor, like bottles and pacifiers. Mop floors and windowsills twice a week. Third, make sure children wash their hands before meals, naptime, and bedtime because these are times when they tend to put their hands in their mouths. Fourth, get professional help to remove lead paint that is peeling or chipping.
Paint over lead paint in good condition to seal it in. Fifth, give children milk with their diet. The calcium in the milk will be absorbed by the body instead of the lead. Children with poor nutrition are more likely to have elevated blood lead levels. Finally, the doctor told Jane to check with her local health department and housing authority for help with her task of minimizing lead exposure. Many government programs and agencies can help educate people about lead poisoning and prevention.
Master 1.5d (page 4 of 6)
Copyright © 2000 by BSCS and Videodiscovery, Inc. Permission granted. Updated 2012.
CASE STUDY #4
The news spread through the school like a wildfire in the spring of 1999. A boy in the eighth grade, Jimmy Green, had died. He had tried for the first time sniffing gasoline from a plastic bag in his garage.
He had a heart attack within minutes of breathing the fumes.
Mr. Cobb, the biology teacher, gathered students and their parents for a meeting. He told them that Jimmy Green died because he inhaled a deadly chemical. He told them that inhaling chemicals, especially ones like gasoline that are made up of so many different chemicals, can be very damaging to the body. Mr.
Cobb reminded students that the lungs can deliver the toxic chemicals that students sniff directly to vital tissues in the body in high concentrations. The results can be short-term memory loss, hearing loss, arm and leg spasms, permanent brain damage, liver and kidney damage, and death.
Mr. Cobb let the families know that Jimmy Green died of “sudden sniffing death syndrome.” In some people, breathing toxic fumes from gasoline can interrupt the normal rhythm of the heart and result in a heart attack. It is impossible to know who might die from sniffing, but 3 out of 10 people who die from sniffing do so during their first use, as Jimmy did. Besides dying from sudden sniffing death syndrome, people who inhale toxic chemicals also die of asphyxia, or suffocation, because of the plastic bags they use; from choking on vomit; and from accidents, fires, and suicides that result from poor decisions made while intoxicated.
Mr. Cobb told the parents that the commercial products that students sniff are easy to get because many people have these products in their homes. In this way, starting a habit of abuse with these chemicals is less expensive than with other substances, such as marijuana or cocaine.
Treatment for people who abuse inhalants is similar to that for people who abuse drugs. It is very difficult to stop using inhalants. Many people who go through rehabilitation to help them stop sniffing need to repeat the program after relapsing back into inhalant use.
Parents and students left the meeting knowing that they did not want to intentionally expose themselves to the dangers of sniffing toxic chemicals. Losing Jimmy Green was enough.
Master 1.5e (page 5 of 6)
Copyright © 2000 by BSCS and Videodiscovery, Inc. Permission granted. Updated 2012.
CASE STUDY #5
In December of 1971, an epidemic occurred in Iraq. More than 6,500 people in many parts of the country became ill with nervous system disorders. They had numbness and tingling around their mouths, lips, fingers, and toes. They had a clumsy, stumbling walk and a feeling of weakness. Some had difficulty swallowing and pronouncing words. Many had vision and hearing loss. In all, 459 people fell into a coma and died.
It took considerable detective work to determine the source of the widespread illness. Finally, it became apparent that a shipment of seed grain from Mexico, which was intended only for planting, was used to make bread instead. The seed had been treated before shipment with a fungicide containing methylmercury (METH-ul-MER-kyoo-ree). The warning about the fungicide and instructions for the seed use were not written in Iraqi, so the people at the southern port of Basra, in Iraq, did not realize the seed should not be eaten. The seed was distributed to households around the country. The women in the country ground the seed to make flour and used it in their baking. The result was mass mercury poisoning from a very unusual source: homemade bread.
Master 1.5f (page 6 of 6)
Copyright © 2000 by BSCS and Videodiscovery, Inc. Permission granted. Updated 2012.
Opening Questions
As a team, think of a chemical that you would like to be exposed to because it makes you healthier. Think of a chemical that you would try to avoid because it harms you in some way. Record these two chemicals in your science notebook.
Use complete sentences to answer the following questions about each of the chemicals:
• Where do you find the chemical?
• How does the chemical enter your body?
• How does it make you healthier or harm you?
• How much chemical do you need to be exposed to for it to help you or harm you?
Master 2.1
Copyright © 2000 by BSCS and Videodiscovery, Inc. Permission granted. Updated 2012.
Making Solutions for Toxicity Testing
Materials for Each Team
6 50-mL beakers, clean and empty
eyedropper
1 50-mL graduated cylinder
masking tape
1 10-mL graduated cylinder
permanent marker
1 100-mL beaker with 50 mL of chemical
safety glasses
100 mL of water in a beaker
latex gloves
1 tray
Procedure
1. Put on the latex gloves and safety glasses.
2. Use the masking tape and marker to label each of the six empty 50-mL beakers with information from the following table.
Beaker #
Amount of
Amount of
Total Volume
% Concentration
Water
Chemical
of Liquid
of Chemical
1
20.00 mL
0.00 mL
20 mL
0%
2
18.75 mL
1.25 mL
20 mL
6.25%
3
17.50 mL
2.50 mL
20 mL
12.5%
4
15.00 mL
5.00 mL
20 mL
25%
5
10.00 mL
10.00 mL
20 mL
50%
6
0.00 mL
20.00 mL
20mL
100%
3. Use the 50-mL and the 10-mL graduated cylinders to measure the correct amount of water. Pour the water into each of the labeled beakers according to the above table. Use the eyedropper for small corrections.
4. Use the 50-mL and the 10-mL graduated cylinders to measure the correct amount of chemical.
Pour the chemical into each of the labeled beakers of water according to the above table. Use the eyedropper for small corrections.
5. When you have finished, check that all the beakers contain 20 mL of chemical solution. If a beaker contains more or less than 20 mL, consult the above table and repeat the procedure for that beaker.
Place the beakers in order on the tray, with 0 percent concentration on the left and 100 percent concentration on the right.
6. Return any unused chemical to your teacher. Wash all other containers and put them away.
Master 2.2
Copyright © 2000 by BSCS and Videodiscovery, Inc. Permission granted. Updated 2012.


Toxicity Testing on Seeds
Materials for Each Team
6 resealable plastic sandwich bags
12 paper napkins
6 beakers of chemical solution, ranging from 0% to 100% concentration
1 bag of seeds (approximately 60 seeds in a bag)
1 permanent marker
latex gloves
safety glasses
1 tray
Procedure
1. Label all six bags with your team members’ initials and a number and a percent concentration of chemical, like this:
Step 1
#1
0% (control)
#2 6.25%
#3 12.5%
#4 25%
#5 50%
#6 100%
2. Put two napkins together and fold them in half so that they fit into the plastic bag. Fill each bag with two folded paper napkins.
Step 2
Step 3
Step 4
3. Put on the safety glasses and latex gloves. Carefully pour the chemical solutions into the bags, making sure to match the numbers and concentration percentages of the bag and the chemical. Each bag now will contain 20 mL of chemical solution that is absorbed by the paper napkins.
Master 2.3a (page 1 of 2)
Copyright © 2000 by BSCS and Videodiscovery, Inc. Permission granted. Updated 2012.
4. Count out 10 seeds. Carefully place the seeds on the moist paper napkins in the control bag (#1), making sure to space them evenly (do not clump them in one spot). Seal the plastic bag, pushing out the air as you go.
5. Repeat Step 4 for the remaining bags.
6. Observe the seeds and fill in the following data table with information you know at this time.
Data table for toxicity testing.
Bag #, Dose
Day 1:
Day 1:
Day 2:
Day 2:
Day 3:
Day 3:
(% concentration)
# of seeds
# of
# of seeds
# of
# of seeds
# of
germinated
seeds not germinated
seeds not germinated
seeds not
germinated
germinated
germinated
1 (control), 0%
2, 6.25%
3, 12.5%
4, 25%
5, 50%
6, 100%
7. Place the seed bags in a stack, lying flat with the seeds up, on the tray. Put the tray of seeds in the spot designated by your teacher. Put this worksheet in your science notebook.
Questions
1. What is your chemical? Describe what you know about the chemical. (Do you consider it harmful, beneficial, or neither? What is it used for? How would a human be exposed to this chemical?) 2. In which bag is the dose of chemical the highest? In which bag is the concentration of chemical in the solution the highest? Describe how you know.
3. Do you think you will see a difference in the effect on seeds of a small dose of chemical compared with the effect of a larger dose? Predict what you think will happen to the seeds in each bag.
Master 2.3b (page 2 of 2)
Copyright © 2000 by BSCS and Videodiscovery, Inc. Permission granted. Updated 2012.
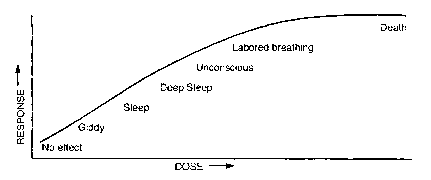
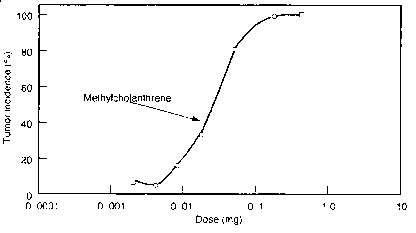
Dose-Response Curves
Response
e
Respons
Dose
Dose
Tumor
incidence
(%)
Dose (mg)
Master 3.1
Copyright © 2000 by BSCS and Videodiscovery, Inc. Permission granted. Updated 2012.
Graph Paper
Name _________________________________________
Date __________________________________________
Class _________________________________________
Directions: Plot dose-response data for your chemical on graph A and for two other teams’ chemicals on graphs B and C.
Chemical A ____________________________________
Master 3.2a (page 1 of 2)
Copyright © 2000 by BSCS and Videodiscovery, Inc. Permission granted. Updated 2012.
Chemical B ____________________________________
Chemical C ____________________________________
Master 3.2b (page 2 of 2)
Copyright © 2000 by BSCS and Videodiscovery, Inc. Permission granted. Updated 2012.
Acetaminophen Dosage Chart
Children’s Chewable Acetaminophen Dosage (80 mg/tablet)
Weight (lb)
Weight (kg)
Age (yr)
Dose
under 24
under 11
under 2
consult doctor
24–35
11–16
2–3
2 tablets
36–47
17–21
4–5
3 tablets
48–59
22–27
6–8
4 tablets
60–71
28–32
9–10
5 tablets
72–95
33–43
11
6 tablets
Warning: Take no more than five doses per day.
Master 4.1
Copyright © 2000 by BSCS and Videodiscovery, Inc. Permission granted. Updated 2012.

A Poisonous Dose? The Case History
Master 4.2a (page 1 of 2)
Copyright © 2000 by BSCS and Videodiscovery, Inc. Permission granted. Updated 2012.

Master 4.2b (page 2 of 2)
Copyright © 2000 by BSCS and Videodiscovery, Inc. Permission granted. Updated 2012.
A Poisonous Dose? The Problem
Your teacher will display a transparency of a doctor’s chart. Read the first page of the doctor’s chart that describes Andy Brown’s case. Then use information provided on the transparency and on this worksheet to answer the following questions.
Part I
How much Infants’ Concentrated Suspension Drops did Andy receive from his aunt?
a. 1 teaspoon
b. 1 teaspoon 4 times in 1 day
c. 3 teaspoons
d. 4 teaspoons
e. b and d
f. none of the above
Use the dosage chart below to calculate how much acetaminophen, in milligrams (mg), Andy’s aunt gave him in one day. Remember, Andy’s aunt gave Andy his medicine in a teaspoon. To use the dosage chart, you need to know that each teaspoon contains 6.25 dropperfuls of medicine.
4 teaspoons x 6.25 dropperfuls x
milligrams = milligrams of acetaminophen
1 teaspoon
1 dropperful
Infants Concentrated Suspension Drops (80 mg/dropper)
Weight (lb)
Weight (kg)
Age (yr)















