4. What is the purpose of KNO3 in this experiment?
5. How would your results for the molar mass of copper be affected if hydrogen gas were also
observed at the cathode?
6. What part of this procedure limits the accuracy of the molar mass determination?
Alchemy - Copper into gold
1. Is this reaction an oxidation-reduction reaction?
1. Why did the penny turn "silver"?
1. Why did it turn "gold"?
1. Why did we heat the penny to turn it "gold"?
Solutions
Chapter 11. Bonding 07
Lab 5: Bonding 07
Objective
To test various compounds and determine their conductivity and bonding.
To understand how electronegativity can predict bond type.
To learn the relationship between bonding and conductivity.
Grading
Pre-Lab (10%)
Lab Report Form (80%)
TA Points (10%)
Background Information
A chemical bond is a link between atoms that results from the mutual attraction of their nuclei for electrons. Bonding occurs in order to lower the total potential energy of each atom or ion.
Throughout nature, changes that decrease potential energy are favored.
The main types of bonds that we will be covering are ionic bonds, covalent bonds, and metallic
bonds. An ionic bond is the chemical bond that results from the electrostatic attraction between positive (cations) and negative (anions) ions. The ionic relationship is a “give and take”
relationship. One ion donates or “gives” electrons, while the other ion receives or “takes”
electrons.
A covalent bond is a chemical bond resulting from the sharing of electrons between two atoms.
There are two main types of covalent bonds. The first being non-polar covalent bonds. These are bonds in which the bonding electrons are shared equally by the united atoms-with a balanced
electrical charge. Polar covalent bonds are covalent bonds in which the united atoms have an
unequal attraction for the shared electrons.
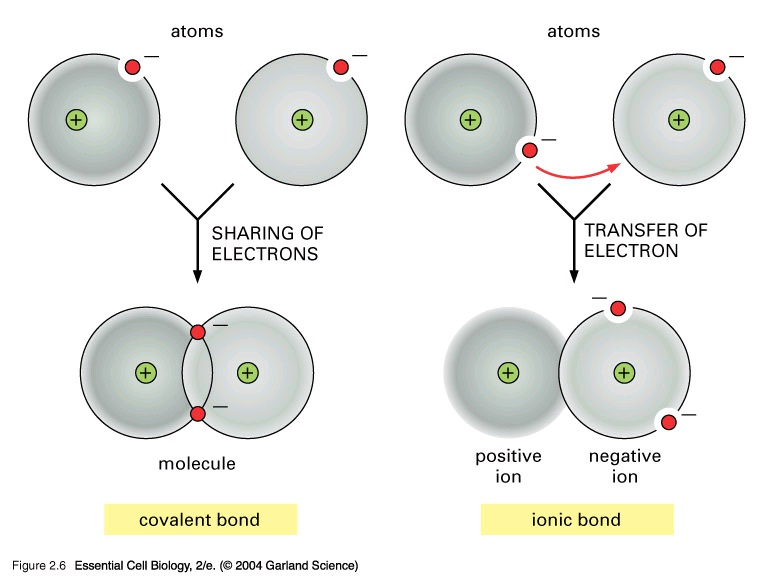
Figure 11.1.
The role of electrons in bonding has been well-studied. The ability of an atom or element to attract electrons to itself is known as the element’s electronegativity. A scale was first calculated by the Nobel laureate Linus Pauling and is commonly called the Pauling electronegativity scale. The
actual electronegativity values aren’t as important as how they compare to a different element. In Part I of today’s experiment, you will compare electronegativity values to predict the type of bond that will exist between two elements.
In the solution state, ionic compounds dissociate to give a separation of charge. The separation of charge allows for the flow of electrons through solution. The flow of electrons is classified as conductivity. A strong electrolyte is a compound that when dissolved in water will completely
ionize or dissociate into ions. That is, the compound exists in water only as individual ions, and there are no intact molecules at all. This solution conducts electricity well. A weak electrolyte is a compound that when dissolved in water only partially ionizes or dissociates into ions. That is, the compound exists in water as a mixture of individual ions and intact molecules. This solution conducts electricity weakly. A nonelectrolyte is a compound that when dissolved in water does not ionize or dissociate into ions at all. In water, this compound exists entirely as intact molecules.
The solution does not conduct electricity at all. By measuring the conductivity of a dissolved
compound, we can classify it as a nonelectrolyte, weak electrolyte, or strong electrolyte and
determine its ability to dissociate into ions. There are four common compounds that you will
encounter in today’s lab.
ACIDS are molecular compounds which ionize (turn into ions) in water. The cation that is formed is always H+. Therefore, in the formulas for simple acids, H is always the first element listed.
Some acids are strong electrolytes and some acids are weak electrolytes. There are no acids which are nonelectrolytes because by definition an acid is a H+ donor.
BASES can be molecular compounds or ionic compounds. Some bases are soluble and some are
not. The soluble bases ionize or dissociate into ions in water, and the anion formed is always
OH−. The ionic bases have hydroxide ( OH− ) as the anion. If they are soluble, the ions simply separate (dissociate) in the water. All of the ionic bases which are soluble are strong electrolytes.
SALTS are ionic compounds which are not acids or bases. In other words, the cation is not
hydrogen and the anion is not hydroxide. Some salts are soluble in water and some are not. All of the salts which are soluble are relatively strong electrolytes.
NONELECTROLYTES are compounds which dissolve in water but do not ionize or dissociate into
ions. These would be molecular compounds other than the acids or bases already discussed.
Experimental Procedure
Caution:Acids and bases are corrosive and can cause burns.
Part I. Predicting bond type through electronegativity differences.
Using the electronegativity table provided in the lab manual, predict the type of bond that each of the following compounds will have by the following process:
Find the electronegativity for each element or ion in compound using electronegativity table
provided.
Subtract the electronegativites (using absolute value).
If values are between:
4.0-1.7---Ionic bond-50-100% ionic
1.7-0.3---Polar Covalent bond-5-50% ionic
0.3-0.0---Non-Polar Covalent-0-5% ionic
Determine the type of bonding in the following compounds: KCl, CO, CaBr2, SiH4, MgS.
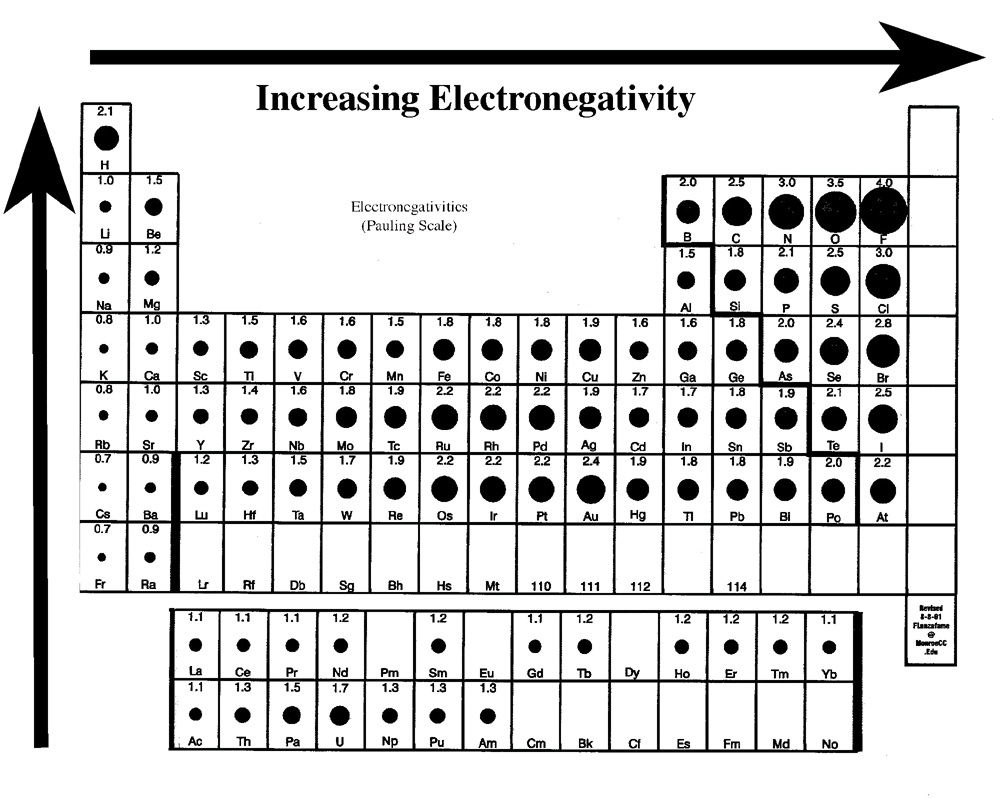
Figure 11.2.
Part II. Weak and strong electrolytes
Chemicals
tap water
0.1 M hydrochloric acid, HCl
0.1 M acetic acid, HC2 H 3 O 2
0.1 M sulfuric acid, H 2SO4
0.1 M sodium hydroxide, NaOH
0.1 M ammonia, NH3
0.1 M sodium acetate, NaC2 H 3 O 2
0.1 M sodium chloride, NaCl
0.1 M ammonium acetate, NH4 C 2 H 3 O 2
0.1 M ammonium chloride, NH4Cl
methanol, CH3OH
ethanol, C 2 H 5OH
sucrose solution, C 12 H 22 O 11
In today’s lab, you will be using a MicroLab conductivity probe to determine how well electrons
flow through a given solution. First, you will need to calibrate the probe with a non-electrolyte (distilled water) and a very strong electrolyte. To quantify how well a solution conducts, we will assign numerical values to the conductance probe. A non-conducting solution will have a
conductance value of 0, a poor conducting solution will have a reading of 0 to 1,000, and good
conductors will have readings of 3,000 up.
Instructions for MicroLab Conductivity Experiment
Open the MicroLab Program by clicking on the Shortcut to MicroLab.exe tab on the desktop.
On the “Choose an Experiment Type” Tab, enter a name for the experiment, and then double click
on the MicroLab Experiment icon
Click “Add Sensor”, Choose sensor = Conductivity Probe
Choose an input, click on the red box that corresponds to the port that your conductivity sensor is connected to. Choose 20,000 microseconds
“Choose a Sensor”, click radial button that says Conductivity Probe. Click next.
Click “Perform New Calibration”
Click “Add Calibration Point” place the conductivity probe in the non-conductive standard
solution, while swirling wait until the value is constant and then enter 0.0 into the “Actual Value”
box in MicroLab and hit “ok”.
Again, Click “Add Calibration Point” place the conductivity probe in the conducting standard
solution, while swirling wait until the value is constant and then enter 1020 into the “Actual
Value” box in MicroLab and hit “ok”. Repeat for 3860 as the Actual Value.
Under Curve Fit Choices , click on “First order (linear)” and then “Accept and Save this
Calibration”, when prompted to “Enter the units for this calibration”, leave as is and click ok, save as your name-experiment-date. Click finish.
In the sensor area, left click on the conductivity icon and drag it to the Y-axis over “data source two”, also click and drag to column B on the spreadsheet and also click and drag to the digital
display window.
When ready to obtain data, click start.
This is very important: Be sure to thoroughly since the probe with DI water between every use.
Beginning with the tap water, measure the conductance of each of the following solutions. Using
the information provided in the lab manual, classify each solution as a non-, weak, or strong
electrolyte. For those solutions that are electrolytes, record the ions present in solution.
Part III. Electrolyte strength and reaction rate
Chemicals
calcium carbonate powder - shake once
1 M HCl - stopper it
1 M HC2 H 3 O 2
0.5 M H 2SO4
Test tube gas collection apparatus - end at 20mL
Measure 2 g of powdered calcium carbonate ( CaCO3) onto a piece of weigh paper. Obtain 30 mL
of 1 M HCl in a graduated cylinder. Pour the acid into the test tube apparatus. Add the calcium
carbonate to the acid and QUICKLY stopper the tube to begin collecting gas. Record the time it
takes to collect 20 mL of gas. The acid may react very fast with the CaCO3 generating the gas very rapidly. Clean out the test tube apparatus and repeat the experiment using 1 M HC2 H 3 O 2 and 0.5
M H 2SO4.
Part IV. Chemical reactions
Chemicals
0.01 M calcium hydroxide, Ca(OH)2
Plastic straws
Plastic straws
Obtain ~20 mL of saturated calcium hydroxide solution. Make sure it is clear and colorless. Place the conductivity probe in the solution and begin monitoring it conductivity. With your straw,
slowly exhale into the solution. Note any observations in the solution and the conductivity.
Pre-Lab 5: Bonding 07
(Total 10 Points)
Hopefully here for the Pre-Lab Name(Print then sign): ___________________________________________________
Lab Day: ___________________Section: ________TA__________________________
This assignment must be completed individually and turned in to your TA at the beginning of lab.
You will not be allowed to begin the lab until you have completed this assignment.
Part I. Bonding of chemicals in solution
1. Write out the formulas of the following acids:
phosphoric ____________________
perchloric ____________________
nitric ____________________
sulfuric __________________
hydrochloric ____________________
acetic ____________________
1. Write out the formulas of the following bases:
calcium hydroxide ____________________
potassium hydroxide ____________________
sodium hydroxide ____________________
ammonia ____________________
1. Write out the formulas of the following salts:
potassium chromate ____________________
potassium sulfate ____________________
copper(II) nitrate ____________________
calcium carbonate ____________________
potassium iodide ____________________
Report 5: Bonding 07
Hopefully here for the Report Form Note: In preparing this report you are free to use references and consult with others. However, you may not copy from other students’ work (including your laboratory partner) or misrepresent your
own data (see honor code).
Name(Print then sign): ___________________________________________________
Lab Day: ___________________Section: ________TA__________________________
Part I. Predicting bond type through electronegativity differences.
Table 11.1.
Chemical Formula Electroneg (1) Electroneg (2) Diff Electroneg Type of bond
KCl
CO
CaBr 2
SiH 4
MgS
Part II. Weak and strong electrolytes
Table 11.2.
Solution Tested
Numerical Output Electrolyte Strength Ions Present
0.1 M HCl
0.1 M HC2 H 3 O 2
0.1 M H 2SO4
0.1 M NaOH
0.1 M NH3
0.1 M NaC2 H 3 O 2
0.1 M NaCl
0.1 M NH4 C 2 H 3 O 2
0.1 M NH4Cl
CH 3 OH
C 2 H 5 OH
Sucrose
Tap water
1. Why do we use deionized water instead of tap water when making solutions for conductivity
measurements?
Part III. Electrolyte strength and reaction rate
2. Time to collect 20 mL of gas using 1 M HCl _______________________. Write the reaction of
HCl with CaCO3.
3. Time to collect 20 mL of gas using 1 M HC2 H 3 O 2_______________________. Write the reaction of HC2 H 3 O 2 with CaCO3.
4. Time to collect 20 mL of gas using 0.5 M H 2SO4_________________________.Write the
reaction of H 2SO4 with CaCO3.
5. Why does it take different lengths of time to collect 20 mL of gas?
6. Based on the time it took to collect 20 mL of gas, rank the acids in the order of increasing
strength.
7. Why did we use 0.5 M H 2SO4 instead of 1.0 M H 2SO4?
Part IV. Chemical reactions
8. Write the chemical reaction for calcium hydroxide with your exhaled breath.
9. Write your observations for the reaction that took place (i.e. appearance, conductivity, etc.) 10. When in separate solutions, aqueous ammonia, NH3(aq) and acetic acid HC2 H 3 O 2 conduct electricity equally well. However, when the two solutions are mixed a substantial increase in
electrical conductivity is observed. Explain.
11. Separately, ammonium sulfate and barium hydroxide solutions are very good conductors.
When the two solutions are mixed a substantial decrease in conductivity is observed. Rationalize this.
Solutions
Chapter 12. Silver Nanoparticles: A Case Study in
Cutting Edge Research
Alvin Orbaek, Mallam Phillips, Dr. Mary McHale, Prof. Andrew Barron,
Objective
To gain an insight into nanotechnology, what it is and how it can be useful, using silver
nanoparticles as an example. We will look at what exactly nanoparticles are, see how they are
made, and how they can be characterized.
The characterization technique involves Ultra-Violet and Visible spectroscopy, so we will look
briefly into the interaction of the nanoparticles and light, which will hopefully help you gain an appreciation for one of the special aspects of nanotechnology.
When making the nanoparticles we will do a time study allowing us to graph the spectroscopic
response - which will show the nature of the particle as it grows, i.e., ripens. We can use some data to calculate the size of the nanoparticle at the beginning and at the end of our experiment.
Background
What is nanotechnology?
Nano is the ancient Greek word for dwarf. In scientific terms it has been used to identify length scales that are one billionth of a unit. This is typically a meter and so you often here things that are nanometers in size. In terms of nanotechnology it has been defined as anything that has a
unique property or function resulting from the size of the artifact being in the nano regime, and that the size regime is between 0.1 and 100 nm. This size range is rather broad; encompassing
simple molecules to more complicated molecules like enzymes. However, these items can be
looked at from many points of view, from a chemist that considers molecules, to that of an
engineer that would look at how each of the molecules interacts in the bigger system and creates new materials from these building blocks. For this reason there are many disciplines that are
interested in the study of nanotechnology such as Chemistry, Physics, Engineering, Biological
sciences, Material Sciences, Computer Science and many more besides. For this reason
nanotechnology is not a strict discipline and many people use their skills and backgrounds from
other areas to contribute to research in this particular field.
Why care about nanotechnology?
There are many effects that occur at the nanoscale that we do not notice on a larger macro scale.
Most of nature actually works at the nanoscale, and by understanding the forces that are at work using knowledge from chemistry, physics and engineering one can better understand the working
of organic life. Enzymes are very large molecules that are too large to consider in terms of
chemistry alone, other effects come into play In order to understand the full picture we need to borrow from physics and computer modeling to gain a better understand of what is happening.
There are many effects that occur at the nanoscale that we do not notice on a larger macro scale.
Most of nature actually works at the nanoscale, and by understanding the forces that are at work using knowledge from chemistry, physics and engineering one can better understand the working
of organic life. Enzymes are very large molecules that are too large to consider in terms of
chemistry alone, other effects come into play In order to understand the full picture we need to borrow from physics and computer modeling to gain a better understand of what is happening.
When we cross from the small scale as in molecules and atoms, to the large scale that we see with our own eyes, we travel through the nanoscale. In that scale we go from quantum physics to
classical physics and a lot of very interesting effects can be used to our benefit, and actually nanoparticles are an excellent example of this. Just by virtue of their size they are able to absorb four times more light than is even shone on them! This is very different from the bulk material, it is difficult to understand in one sitting, but let’s just say that there is a coupling between the light energy and the matter of the nanoparticles that is best explained through quantum mechanics, but we won’t go into that now.
When you make something very large, there is lots of room for error, the more parts you have in a system the more chances there are that some of those parts can be faulty. However when you make
something in the nanoscale you have far less parts in the system and each part has to be virtually perfect. Material scientists are concerned with the defects that are created in materials, because these are the parts that cause a material to break down often and stop functioning correctly. As you get into the nanoscale there are less defects and you get enhanced effects from the purer
material, that don’t occur on the larger scale. One example of this is carbon nanotubes, by virtue of there shape and size they are 6 times lighter than steel, but almost 100 times stronger. There is great potential for using these in new materials in the future that are ultra lightweight and
extremely strong.
When we make things with modern technology we have for centuries been using a top down
approach, and this brings us down to a fine limit but not as fine as that on which nature works.
Nanotechnology is more about understanding the fundamental forces in nature by physics, and
seeing their interaction through chemistry, and then making something larger from our
engineering skills. And we can always take examples from biology that has been doing this for far longer than we have. So really what we do is take a bottom up approach, so that we can create
large materials that we can use, that has every part of the interaction tailored all the way from how the atoms interact and how the molecules are formed and bonded together to make building blocks
for new materials and applications. This bottom up approach is a change in the way things have
been done and for this reason nanotechnology is a very potent discipline, with an immense
capacity for expansion.
In all we have only really begun to scratch the surface of what could be possible when we create things using nanotechnology, and we should be aware of this because nanotechnology is finding
its way into every corner of life, from health studies, medicine, robotics, materials and maybe
even food and many many more.
What are nanoparticles and how are they made?
A simple way of seeing this is by imagining tennis balls that are squeezed down to a few billionths of a meter. The particles are rounded because they try to minimize the surface energy as much as possible; any edges will make things more energetic since typically nature follows the path of
least resistance the particles tend to form colloids, or spheres with as few edges as possible. It is possible though, to direct the growth of nanoparticles into various shapes such as cubes, and
tetrahedrons. We will concern ourselves with only colloidal nanoparticles for the moment.
The nanoparticles have a large surface area compared with the total volume. The surface area to
volume ratio is interesting because chemical reactions typically occur on surfaces, so
nanoparticles that have a high surface to energy ratio can be used in many interesting ways, such as in catalysis. One teaspoon of nanoparticles might weigh only 200 mg, but because of their
shape and the large amount of surface area the tea spoon could have the same surface area as a
whole football field! This gives them huge potential and potency compared to the bulk material.
Imagine laying out a football field with a thin layer of silver, think how much silver that would need, and then compare that with the amount that is in the spoon! This high surface area to volume ratio is one of the most important properties about nanoparticles.
With all that surface area and the energy that exists, the nanoparticles need to be held together
‘somehow’. That is where the furry parts of the tennis ball come into play. Imagine them as small molecules that hold on to the surface of the particle and stop it from breaking up under its own energy. It is like a tree whose roots can prevent soil erosion because the soil is bonded to the root in the ground. The chemical we use in this lab is mercaptosuccinic acid, and this helps to hold the nanoparticles in shape by bonding to the surface of the particles.
There are a few basic points to remember about making nanoparticles:
1) You need a nucleation point, a place for the metal (silver in this case) to start bonding to one another and start growing into a larger particle. For this you often need some ingredient that can break down a metal salt, in this case silver nitrate, which is accomplished by using sodium
borohydride. This reduces the silver nitrate into silver ions that are free then to bond with each other.
2) You need some mechanism to keep the particles at the nanoscale and stop them from ripping
and growing into something much larger, this is accomplished using the capping agent mentioned
earlier (mercaptosuccinic acid). A great deal of cutting edge research revolves around varying the capping agent in order to control the size of your nanoparticles and tailor them for specific tasks.
But not only can you change the size of particles in this way, you can also change the shapes.
Why silver nanoparticles?
Silver is a very easily oxidized material; it has been used already commercially for its anti-
microbial properties from athletic wear to sterilizing water. It has a very interesting interaction with light du





