



shell electron pair description of the bonding in H 2. Each hydrogen atom has a single electron. By sharing these two electrons, each hydrogen atom can fill its valence shell, attaining the electron
configuration of helium.
How does this translate into the electron orbital picture of electron sharing that we have just
described for the
molecular ion? There are two ways to deduce the answer to this question,
and, since they are both useful, we will work through them both. The first way is to imagine that
we form an H 2 molecule by starting with an
molecular ion and adding an electron to it. As a
simple approximation, we might imagine that the first electron’s probability distribution (its
orbital) is not affected by the addition of the second electron. The second electron must have a
probability distribution describing its location in the molecule as well. We recall that, in atoms, it
is possible to put two electrons into a single electron orbital, provided that the two electrons have
opposite values of the spin quantum number, ms. Therefore, we expect this to be true for
molecules as well, and we place the added second electron in H 2 into the same σ orbital as the first. This results in two electrons in the region between the two nuclei, thus adding to the force of
attraction of the two nuclei into the bond. This explains our observation that the bond energy of H 2
is almost (although not quite) twice the bond energy of
.
The second way to understand the electron orbital picture of H 2 is to imagine that we form the
molecule by starting with two separated hydrogen atoms. Each of these atoms has a single
electron in a 1s orbital. As the two atoms approach one another, each electron orbital is polarized
in the direction of the other atom. Once the atoms are close enough together, these two orbitals
become superimposed. Now we must recall that these orbitals describe the wave-like motion of
the electron, so that, when these two wave functions overlap, they must interfere, either
constructively or destructively. In Fig. 3, we see the consequences of constructive and destructive
interference. We can deduce that, in H 2 the electron orbitals from the atoms must constructively interfere, because that would increase the electron probability in the region between the nuclei,
resulting in bonding as before. Therefore, the σ molecular orbital describing the two electrons in H 2 can be understood as resulting from the constructive overlap of two atomic 1s electron orbitals.
We now add to our observations of diatomic molecules by noting that, of the diatomic molecules
formed from like atoms of the first ten elements, H 2, , B 2, C 2, N 2, O 2, and F 2 are stable molecules with chemical bonds, whereas
,
, and
are not bound. In examining the
electron configurations of the atoms of these elements, we discover a correspondence with which
diatomic molecules are bound and which ones are not.
all have odd numbers of
electrons, so that at least one electron in each atom is unpaired. By contrast, He, Be, and Ne all
have even numbers of electrons, none of which are unpaired. The other atoms, C and O both have
an even number of electrons. However, as deduced in our understanding of the electron
configurations in atoms, electrons will, when possible, distribute themselves into different orbitals
of the same energy so as to reduce the effect of their mutual repulsion. Thus, in C and O, there are
three 2p orbitals into which 2 and 4 electrons are placed, respectively. Therefore, in both atoms,
there are two unpaired electrons. We conclude that bonds will form between atoms if and only if






there are unpaired electrons in these atoms.
In H 2, the unpaired electrons from the separated atoms become paired in a molecular orbital
formed from the overlap of the 1s atomic electron orbitals. In the case of a hydrogen atom, then,
there are of course no paired electrons in the atom to worry about. In all other atoms, there
certainly are paired electrons, regardless of whether there are or are not unpaired electrons. For
example, in a lithium atom, there are two paired electrons in a 1s orbital and an unpaired electron
in the 2s orbital. To form
, the unpaired electron from each atom can be placed into a molecular
orbital formed from the overlap of the 2s atomic electron orbitals. However, what becomes of the
two electrons paired in the 1s orbital in a Li atom during the bonding of
?
To answer this question, we examine
, in which each atom begins with only the two 1s
electrons. As we bring the two He atoms together from a large distance, these 1s orbitals should
become polarized, as in the hydrogen atom. When the polarized 1s orbitals overlap, constructive
interference will again result in a σ molecular orbital, just as in H 2. Yet, we observe that is not
a stable bound molecule. The problem which prevents bonding for
arises from the Pauli
Exclusion Principle: only two of the four electrons in
can be placed into this σ bonding
molecular orbital. The other two must go into a different orbital with a different probability
distribution. To deduce the form of this new orbital, we recall that the bonding orbital discussed
so far arises from the constructive interference of the atomic orbitals, as shown in Fig. 3. We
could, instead, have assumed destructive interference of these orbitals. Destructive interference of
two waves eliminates amplitude in the region of overlap of the waves, also shown in Fig. 3. In the
case of the atomic orbitals, this means that the molecular orbital formed from destructive
interference decreases probability for the electron to be between in the nuclei. Therefore, it
increases probability for the electron to be outside the nuclei, as in Fig. 1a. As discussed there,
this arrangement for the electron does not result in bonding; instead, the nuclei repel each other
and the atoms are forced apart. This orbital is thus called an anti-bonding orbital. This orbital also
has the symmetry of a cylinder along the bond axis, so it is also a σ orbital; to indicate that it is an anti-bonding orbital, we designate it with an asterisk, σ*
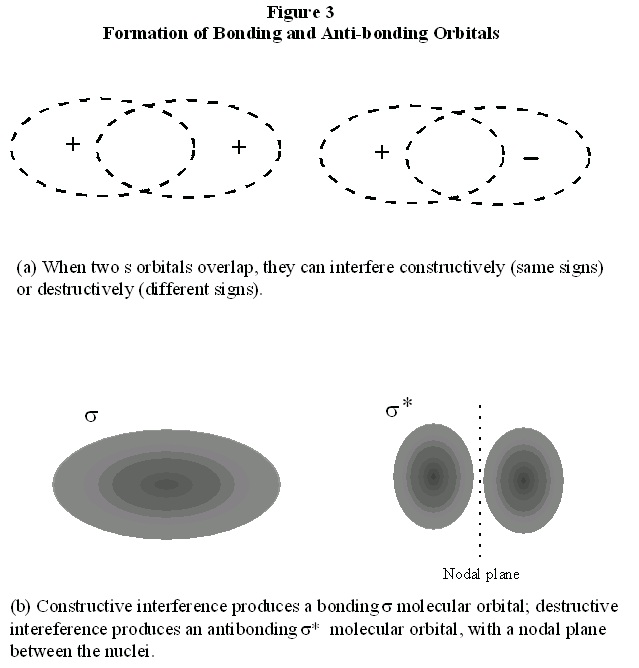


Figure 8.3.
In
, both the bonding and the anti-bonding orbitals must be used in order to accommodate four
electrons. The two electrons in the bonding orbital lower the energy of the molecule, but the two
electrons in the anti-bonding orbital raise it. Since two He atoms will not bind together, then the
net effect must be that the anti-bonding orbital more than offsets the bonding orbital.
We have now deduced an explanation for why the paired electrons in an atom do not contribute to
bonding. Both bonding and anti-bonding orbitals are always formed when two atomic orbitals
overlap. When the electrons are already paired in the atomic orbitals, then there are too many
electrons for the bonding molecular orbital. The extra electrons must go into the anti-bonding
orbital, which raises the energy of the molecule, preventing the bond from forming.
Returning to the
example discussed above, we can develop a simple picture of the bonding.
The two 1s electrons from each atom do not participate in the bonding, since the anti-bonding
more than offsets the bonding. Thus, the paired “core” electrons remain in their atomic orbitals,
unshared, and we can ignore them in describing the bond. The bond is formed due to overlap of

the 2s orbitals and sharing of these electrons only. This is also consistent with our earlier view
that the core electrons are closer to the nucleus, and thus unlikely to be shared by two atoms.
The model we have constructed seems to describe fairly well the bonding in the bound diatomic
molecules listed above. For example, in a fluorine atom, the only unpaired electron is in a 2p
orbital. Recall that a 2p orbital has two lobes, directed along one axis. If these lobes are assumed
to lie along the axis between the two nuclei in F 2, then we can overlap them to form a bonding
orbital. Placing the two unpaired electrons into this orbital then results in a single shared pair of
electrons and a stable molecular bond.
Observation 3: Ionization energies of diatomic molecule
The energies of electrons in molecular orbitals can be observed directly by measuring the
ionization energy. This is the energy required to remove an electron, in this case, from a molecule:
The measured ionization energy of H 2 is 1488 kJ/mol. This number is primarily important in
comparison to the ionization energy of a hydrogen atom, which is 1312 kJ/mol. Therefore, it
requires more energy to remove an electron from the hydrogen molecule than from the hydrogen
atom, so we can conclude that the electron has a lower energy in the molecule. If we attempt to
pull the atoms apart, we must raise the energy of the electron. Hence, energy is required to break
the bond, so the molecule is bound.
We conclude that a bond is formed when the energy of the electrons in the molecule is lower than
the energy of the electrons in the separated atoms. This conclusion seems consistent with our
previous view of shared electrons in bonding molecular orbitals.
As a second example, we consider the nitrogen molecule, N 2. We find that the ionization energy of molecular nitrogen is 1503 kJ/mol, and that of atomic nitrogen is 1402 kJ/mol. Once again, we
conclude that the energy of the electrons in molecular nitrogen is lower than that of the electrons
in the separated atoms, so the molecule is bound.
As a third example, we consider fluorine, F 2. In this case, we find that the ionization energy of molecular fluorine is 1515 kJ/mol, which is smaller than the ionization energy of a fluorine atom,
1681 kJ/mol. This seems inconsistent with the bonding orbital concept we have developed above,
which states that the electrons in the bond have a lower energy than in the separated atoms. If the
electron being ionized has a higher energy in F 2 than in F, why is F 2 a stable molecule?
Apparently, we need a more complete description of the molecular orbital concept of chemical
bonding.
To proceed further, we compare bond energies in several molecules. Recall that the bond energy
(or bond strength) is the energy required to separate the bonded atoms. We observe that the bond
energy of N 2 is 956 kJ/mol. This is very much larger than the bond energy of H 2, 458 kJ/mol, and of F 2, which is 160 kJ/mol. We can account for the unusually strong bond in nitrogen using both our valence shell electron pair sharing model and our electron orbital descriptions. A nitrogen
atom has three unpaired electrons in its valence shell, because the three 2p electrons distribute
themselves over the three 2p orbitals, each oriented along a different axis. Each of these unpaired
electrons is available for sharing with a second nitrogen atom. The result, from valence shell
electron pair sharing concepts, is that three pairs of electrons are shared between two nitrogen
atoms, and we call the bond in N 2 a “triple bond.” It is somewhat intuitive that the triple bond in N 2 should be much stronger than the single bond in H 2 or in F 2.
Now consider the molecular orbital description of bonding in N 2. Each of the three 2p atomic
orbitals in each nitrogen atom must overlap to form a bonding molecular orbital, if we are to
accommodate three electron pairs. Each 2p orbital is oriented along a single axis. One 2p orbital
from each atom is oriented in the direction of the other atom, that is, along the bond axis. When
these two atomic orbitals overlap, they form a molecular orbital which has the symmetry of a
cylinder and which is therefore a σ orbital. Of course, they also form a σ*orbital. The two electrons are then paired in the bonding orbital.
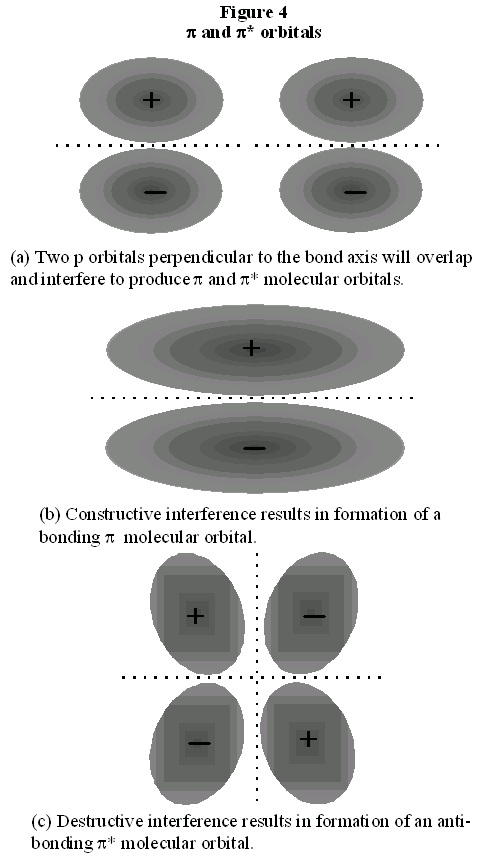
Figure 8.4.
The other two 2p orbitals on each nitrogen atom are perpendicular to the bond axis. The
constructive overlap between these orbitals from different atoms must therefore result in a
molecular orbital somewhat different that what we have discussed before. As shown in Fig. 4, the
molecular orbital which results now does not have the symmetry of a cylinder, and in fact, looks
something more like a cylinder cut into two pieces. This we call a π orbital. There are two such π
orbitals since there are two sets of p orbitals perpendicular to the bond axis. Figure 4 also shows
that an anti-bonding orbital is formed from the destructive overlap of 2p orbitals, and this is called
a π* orbital. There are also two π* orbitals formed from destructive overlap of 2p orbitals. In N 2, the three shared electron pairs are thus in a single σ orbital and in two π orbitals. Each of these orbitals is a bonding orbital, therefore all six electrons have their energy lowered in comparison to
the separated atoms.
This is depicted in Fig. 5 in what is called a “molecular orbital energy diagram.” Each pair of
atomic orbitals, one from each atom, is overlapped to form a bonding and an anti-bonding orbital.
The three 2p orbitals from each atom form one σ and σ* pair and two π and π* pairs. The lowering of the energies of the electrons in the σ and π orbitals is apparent. The ten n=2 electrons from the nitrogen atoms are then placed pairwise, in order of increasing energy, into these molecular
orbitals. Note that, in agreement with the Pauli Exclusion Principle, each pair in a single orbital
consists of one spin up and one spin down electron.
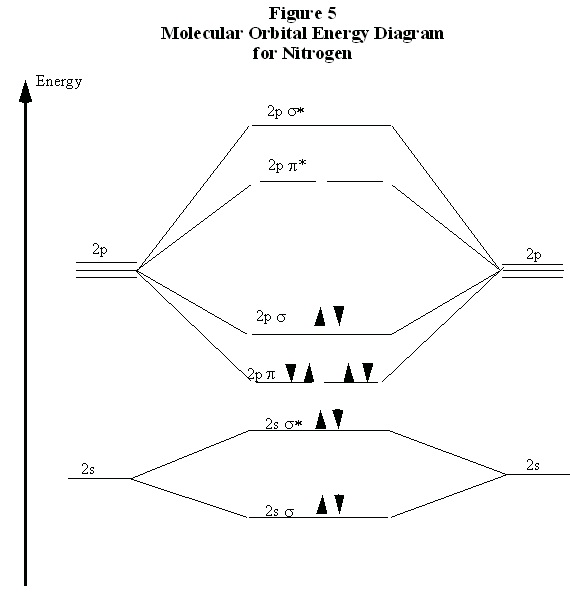
Figure 8.5.
Recall now that we began the discussion of bonding in N 2 because of the curious result that the ionization energy of an electron in F 2 is less than that of an electron in an F atom. By comparing the molecular orbital energy level diagrams for N 2 and F 2 we are now prepared to answer this puzzle. There are five p electrons in each fluorine atom. These ten electrons must be distributed
over the molecular orbitals whose energies are shown in Fig. 6. (Note that the ordering of the
bonding 2p orbitals differ between N 2 and F 2.) We place two electrons in the σ orbital, four more in the two π orbitals, and four more in the two π* orbitals. Overall, there are six electrons in bonding orbitals and four in anti-bonding orbitals. Since F 2 is a stable molecule, we must
conclude that the lowering of energy for the electrons in the bonding orbitals is greater than the
raising of energy for the electrons in the antibonding orbitals. Overall, this distribution of
electrons is, net, equivalent to having two electrons paired in a single bonding orbital.
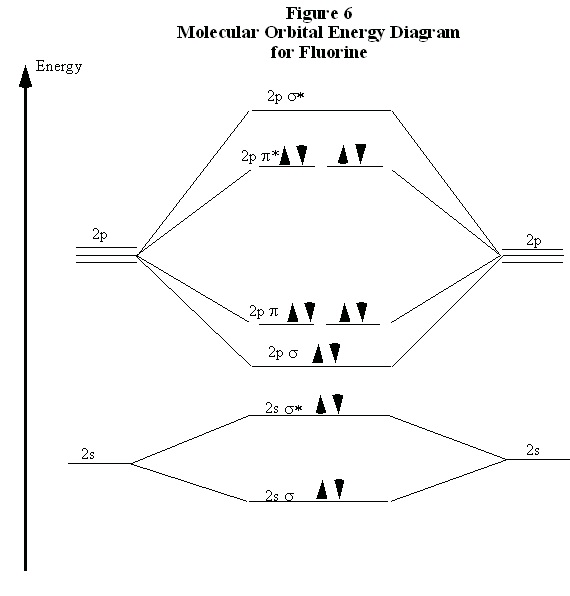
Figure 8.6.
This also explains why the ionization energy of F 2 is less than that of an F atom. The electron with the highest energy requires the least energy to remove from the molecule or atom. The molecular
orbital energy diagram in Fig. 6 clearly shows that the highest energy electrons in F 2 are in antibonding orbitals. Therefore, one of these electrons is easier to remove than an electron in an
atomic 2p orbital, because the energy of an anti-bonding orbital is higher than that of the atomic
orbitals. (Recall that this is why an anti-bonding orbital is, indeed, anti-bonding.) Therefore, the
ionization energy of molecular fluorine is less than that of atomic fluorine. This clearly
demonstrates the physical reality and importance of the anti-bonding orbitals.
A particularly interesting case is the oxygen molecule, O 2. In completing the molecular orbital energy level diagram for oxygen, we discover that we must decide whether to pair the last two
electrons in the same 2pπ* orbital, or whether they should be separated into different 2pπ* orbitals.
To determine which, we note that oxygen molecules are paramagnetic, meaning that they are
strongly attracted to a magnetic field. To account for this paramagnetism, we recall that electron
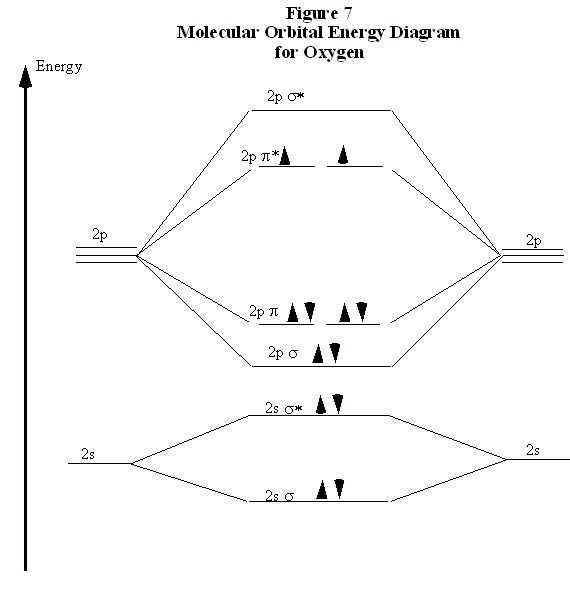
spin is a magnetic property. In most molecules, all electrons are paired, so for each “spin up”
electron there is a “spin down” electron and their magnetic fields cancel out. When all electrons
are paired, the molecule is diamagnetic meaning that it responds only weakly to a magnetic field.
If the electrons are not paired, they can adopt the same spin in the presence of a magnetic field.
This accounts for the attraction of the paramagnetic molecule to the magnetic field. Therefore, for
a molecule to be paramagnetic, it must have unpaired electrons. The correct molecular orbital
energy level diagram for an O 2 molecule is shown in Fig. 7.
Figure 8.7.
In comparing these three diatomic molecules, we recall that N 2 has the strongest bond, followed by O 2 and F 2. We have previously accounted for this comparison with Lewis structures, showing that N 2 is a triple bond, O 2 is a double bond, and F 2 is a single bond. The molecular orbital energy level diagrams in Figs. 5 to 7 cast a new light on this analysis. Note that, in each case, the number
of bonding electrons in these molecules is eight. The difference in bonding is entirely due to the








number of antibonding electrons: 2 for N 2 , 4 for O 2 , and six for F 2. Thus, the strength of a bond must be related to the relative numbers of bonding and antibonding electrons in the molecule.
Therefore, we now define the bond order as
Note that, defined this way, the bond order for N 2 is 3, for O 2 is 2, and for F 2 is 1, which agrees with our conclusions from Lewis structures. We conclude that we can predict the relative
strengths of bonds by comparing bond orders.
Review and Discussion Questions
1. Why does an electron shared by two nuclei have a lower potential energy than an electron on a
single atom? Why does an electron shared by two nuclei have a lower kinetic energy than an
electron on a single atom? How does this sharing result in a stable molecule? How can this
affect be measured experimentally?
2. Explain why the bond in an H 2 molecule is almost twice as strong as the bond in the
ion.
Explain why the H 2 bond is less than twice as strong as the
bond.
3. Be2 is not a stable molecule. What information can we determine from this observation about
the energies of molecular orbitals?
4. Less energy is required to remove an electron from an F 2 molecule than to remove an electron from an F atom. Therefore, the energy of that electron is higher in the molecule than in the
atom. Explain why, nevertheless, F 2 is a stable molecule, i.e., the energy of an F 2 molecule is less than the energy of two F atoms.
5. Why do the orbitals of an atom "hybridize" when forming a bond?
6. Calculate the bond orders of the following molecules and predict which molecule in each pair
has the stronger bond:
1. C 2 or
2. B 2 or
3. F 2 or
4. O 2 or
7. Which of the following diatomic molecules are paramagnetic: CO,
, NO, N 2 ?
8. B 2 is observed to be paramagnetic. Using this information, draw an appropriate molecular
orbital energy level diagram for B 2.
Solutions
Chapter 9. Energetics of Chemical Reactions
The Foundation
We begin our study of the energetics of chemical reactions with our understanding of mass
relationships, determined by the stoichiometry of balanced reactions and the relative atomic
masses of the elements. We will assume a conceptual understanding of energy based on the
physics of mechanics, and in particular, we will assume the law of conservation of energy. In
developing a molecular understanding of the reaction energetics, we will further assume our
understanding of chemical bonding via valence shell electron pair sharing and molecular orbital
theory.
Goals
The heat released or consumed in a chemical reaction is typically amongst the most easily
observed and most readily appreciated consequences of the reaction. Many chemical reactions are
performed routinely specifically for the purpose of utilizing the heat released by the reaction.
We are interested here in an understanding of the energetics of chemical reactions. Specifically,
we wish to know what factors determine whether heat is absorbed or released during a chemical
reaction. With that knowledge, we seek to quantify and predict the amount of heat anticipated in a
chemical reaction. We expect to find that the quantity of heat absorbed or released during a
reaction is related to the bonding of the molecules involved in the reaction.
Prior to answering these questions, we must first answer a few questions regarding the nature of
heat. Despite our common familiarity with heat (particularly in Houston), the concept of heat is
somewhat elusive to define. We recognize heat as "whatever it is that makes things hot," but this definition is too imprecise to permit measurement or any other conceptual progress. Exactly how
do we define and measure heat?
Observation 1: Measurement of Heat by Temperature
We can define in a variety of ways a temperature scale which permits quantitative measurement
of "how hot" an object is. Such scales are typically based on the expansion and contraction of materials, particularly of liquid mercury, or on variation of resistance in wires or thermocouples.
Using such scales, we can easily show that heating an object causes its temperature to rise.
It is important, however, to distinguish between heat and temperature. These two concepts are not
one and the same. To illustrate the difference, we begin by measuring the temperature rise
produced by a given amount of heat, focusing on the temperature rise in 1000g of water produced


by burning 1.0g of methane gas. We discover by performing this experiment repeatedly that the
temperature of this quantity of water always rises by exactly 13.3°C. Therefore, the same quantity
of heat must alway































