A number of oxidising agents may be used. Acidified sodium dichromate (VI) solution at room
temperature will oxidise primary alcohols to aldehydes and secondary alcohols to ketones. At
higher temperatures primary alcohols are oxides further to acids.
Figure 9.1.
The dichromate solution turns from the orange color of the
(aq) to the blue color of the
(aq). This color change is the basis for the "breathalyser test". The police can ask a motorist to exhale through a tube containing some orange crystals. If the crystals turn blue, it shows that the breath contains a considerable amount of ethanol vapor.
Soaps are produced by the reaction of metallic hydroxides with animal fats and vegetable oils. The major components of these fats and oils are triglycerides. Triglycerides are esters of the
trihydroxy alcohol called glycerol and various long-chain fatty acids. Tristearin is a typical
triglyceride. Upon reaction with sodium hydroxide, the ester bonds of tristearin are broken. The products of the reaction are the soap, sodium stearate, and glycerol. This type of reaction is called saponification (Greek: sapon, soap) and it is depicted below.
Figure 9.2.
Soap is made commercially by heating beef tallow in large kettles with an excess of sodium
hydroxide. When sodium chloride is added to this mixture (called the "saponified" mixture), the sodium salts of the fatty acids separate as a thick curd of crude soap. Glycerol is an important by-product of the reaction. It is recovered by evaporating the water layer. The crude soap is purified,



and coloring agents and perfumes are added to meet market demands.
EXPERIMENTAL PROCEDURE
CAUTION WEAR EYE PROTECTION!
CAUTION - Concentrated sulfuric acid will burn and stain the skin as well as damage clothing. In case of skin or clothing contact, wash the area immediately with large amounts of water.
Synthesis of esters
1. Place approximately 2 g (or 2 mL if the substance is a liquid) of the organic acid and 2 mL of the alcohol in a large test tube.
2. Add 5 - 7 drops of concentrated (18 M) sulfuric acid, mix the solution well with a glass
stirring rod and then place the test tube in a hot water bath (largest beaker in your drawer) (~
80°C) for 5 - 10 minutes.
3. Remove the test tube from the hot water bath and cautiously pour the mixture into about 15
mL of saturated sodium bicarbonate contained in a small beaker. The sodium bicarbonate will
destroy any unreacted acid.
4. Observe the aroma produced from each of the following esterification reactions. Write the
structure of the esters produced, and the balanced equations for the esterification and the
acid/sodium bicarbonate reactions:
Complete the following reactions using the procedure above and record your observations.
(1)
salicylic acid + methyl alcohol
(2)
1 - octanol + glacial acetic acid
(3)

amyl alcohol + glacial acetic acid
(4)
ethanol + acetic acid
Oxidation of an alcohol with acidified potassium dichromate(VI) solution
Add 10 drops of dilute sulfuric acid (6M) and 5 drops of potassium dichromate(VI) solution
(0.01M) to 5 drops of ethanol. The oxidising agent is added slowly to the alcohol so that the
temperature is kept below that of the alcohol and above that of the carbonyl compound.
(Carbonyl compounds are more volatile than the corresponding alcohols). Usually the alcohol
is in excess of the oxidant and the aldehyde is distilled off to avoid further oxidation.
Note the color and smell cautiously (Royal Wave).
Warm the mixture and smell cautiously (Royal Wave).
Repeat the experiment using first methanol and then propan-2-ol in place of ethanol.
Describe what happens and explain the color changes.
What conditions and techniques would favour the oxidation of ethanol to
a. ethanal rather than ethanoic acid.
b. ethanoic acid rather than ethanal?
Oxidation of an alcohol with acidified potassium permanganate (VII) solution
Add 10 drops of dilute sulfuric acid and 5 drops of potassium permanganate (VII) solution
(0.01M) to 5 drops of ethanol. Note the color and smell cautiously.
Warm the mixture and smell cautiously (Royal Wave).
Repeat the experiment using first methanol and then propan-2-ol in place of ethanol.
Take the pH of your final mixture using Universal indicator paper
Describe what happens and explain the color changes.
What is your final product?
Saponification of a vegetable oil
CAUTION - Sodium hydroxide is a very caustic material that can cause severe skin burns. Eye
burns caused by sodium hydroxide are progressive: what at first appears to be a minor irritation can develop into a severe injury unless the chemical is completely flushed from the eye. If sodium hydroxide comes in contact with the eye, flush the eye with running water continuously for at least 20 minutes. Notify your TA immediately. If sodium hydroxide is spilled on some other parts of
the body, flush the affected area with running water continuously for at least 2-3 minutes. Notify your TA immediately.
Never handle sodium hydroxide pellets with your fingers. Use weighing paper and a scoopula.
Solid sodium hydroxide will absorb water from the atmosphere. It is hygroscopic. Do not leave
the container of sodium hydroxide open.
Keep ethanol and ethanol-water mixtures away from open flames.
Aqueous iron chloride will stain clothes permanently and is irritating to the skin. Avoid contact with this material.
In this experiment, you will saponify a vegetable oil
1. Pour 5 mL (5.0 g) of vegetable oil into a 250-mL beaker.
2. Slowly dissolve 2.5 g of NaOH pellets in 15 mL of the 50% ethanol/water mixture in a 50-mL
beaker.
3. Add 2-3 mL of the NaOH solution to the beaker containing the oil. Heat the mixture over a hot plate with stirring. CAUTION: Keep your face away from the beaker and work at arm's length.
Stirring is required to prevent spattering. Every few minutes, for the next 20 minutes, add
portions of the ethanol/water mixture while continuing to stir to prevent spattering. After
about 10 more minutes of heating and stirring, the oil should be dissolved and a homogenous
solution should be obtained.
4. Add 25 mL of water to the hot solution. Using the hot grips, pour this solution into a 250 mL
beaker containing 150 mL of saturated NaCl solution. Stir this mixture gently and permit it to
cool for a few minutes.
5. Skim the soap layer off the top of the solution and place it in a 50-mL beaker.
6. Into a test tube, place a pea-sized lump of your soap. Place a similar amount of laundry

detergent in a second tube and a similar amount of laundry detergent in a second tube and a
similar amount of hand soap in a third tube. Add 10 mL of water to each tube. Stopper each
tube and shake thoroughly.
7. Estimate the pH of the solution using wide-range indicator solution or wide-range test paper.
Record the results. Pour the contents of the test tubes into the sink and rinse the tubes with
water.
Figure 9.3.
Pre-Lab: Introductory Organic Reactions
(Total 25 Points)
Hopefully here for the Pre-Lab Name(Print then sign): ___________________________________________________
Lab Day: ___________________Section: ________TA__________________________
This assignment must be completed individually and turned in to your TA at the beginning of lab.
You will not be allowed to begin the lab until you have completed this assignment.
For questions 1-4, draw the structural formulae of:
1) 2,2 - dimethylbutane
2) 3-ethyl-2,4-dimethylpentane
3) 2,3,4-trimethylhexane




4) 3-ethyl-2-methylheptane
For questions 5-8, give the names of
5)
6)
7)
8)
For questions 9-11, give the structural formulae for:
9) hex-3-ene
10) 3-methylhex-1-ene



11) 2,5- dimethylhex-2-ene
For questions 12-14, give the names of:
12)
Figure 9.4.
13)
Figure 9.5.
14)
Figure 9.6.
For questions 15-19, give the stuctural formulae of:
15) trans-1,2-dibromoethene
16) trans-1-chloroprop-1-ene
17) cis- hex-2-ene




18) pent-1-yne
19) 3-methylbut-1-yne
For questions 20-25, name the following compounds:
20)
Figure 9.7.
21)
Figure 9.8.
22)
Figure 9.9.
23)
Figure 9.10.
24)







Figure 9.11.
25)
Figure 9.12.
Report: Organic Reactions
Hopefully here for the Report Form Note: In preparing this report you are free to use references and consult with others. However, you may not copy from other students’ work (including your laboratory partner) or misrepresent your own data (see honor code).
Name(Print then sign): ___________________________________________________
Lab Day: ___________________Section: ________TA__________________________
Observations:
Synthesis of esters
Table 9.1.
Reagents
Product Observations
C 7 H 6 O 3(salicylic acid ) +
(methyl alcohol)
(1 - octanol )
(glacial acetic acid)
(amyl alcohol)
(glacial acetic acid)






(ethanol)
(acetic acid)
Oxidation of an alcohol with acidified potassium dichromate(VI) solution.
Remember to describe what happens and explain the color changes.
What conditions and techniques would favour the oxidation of ethanol to
a. ethanal rather than ethanoic acid.
b. ethanoic acid rather than ethanal?
Table 9.2.
Observations:
Add 10 drops of dil.
to 5 drops of
Observations: color/smell on
color/smellSmell
to the following alcohols
warmingSmell cautiously!
cautiously!
Ethanol
Methanol
Propan-2-ol
Oxidation of an alcohol with acidified potassium permanganate (VII) solution
Remember to describe what happens and explain the color changes.
What is your final product?
Table 9.3.
Observations:
Add 10 drops of dil.
to 5 drops
Observations: color/smell on
color/smellSmell
of
to the following alcohols
warmingSmell cautiously!
cautiously!
Ethanol
Methanol
Propan-2-ol
Saponification of a vegetable oil
Table 9.4.
Reagent
pH from indicator paper
Your soap
Laundry Detergent
Hand Soap
w from the File menu, and then double-click your template.
Solutions



Chapter 10. Transition Metals
Transitions Metals: Synthesis of an Inorganic Compound (trans-
dinitrobis(ethylenediamine)cobalt(III) nitrate)
Objectives
To synthesize a transition metal complex of cobalt three, Co(III), and ethylenediamine.
To characterize the resulting metal complex spectroscopically.
To understand concept of limiting reactant.
Grading
Your will be determined according to the following:
prelab (10%)
lab report form (80%)
TA points (10%)
Introduction
The transition metals are the largest “group” (classification) of elements from the periodic table.
These can be found in nature as ores or in its elemental form, such as gold. All transition metals have more than one oxidation state. Most transition metals (TMs) can complex with other species (called ligands in “TM Complex” jargon) by giving their electrons to them, forming a complex.
These ligands, which are the nearest neighbor atoms to the metal center, constitute the inner (or first) coordination sphere. Complexes may be either neutral or charged and have distinctive
properties that may be quite unlike those associated with their constituent molecules and ions, each of which is capable of independent existence. An example of a charged complex is
ferricyanide,
. The
and
ions found in the ferricyanide complex ion exist as
independent species and in other compounds. The transition metals are well known for forming a
large number of complex ions. In this experiment we will synthesize a transition metal complex
containing cobalt, Co(III), and ethylenediamine.
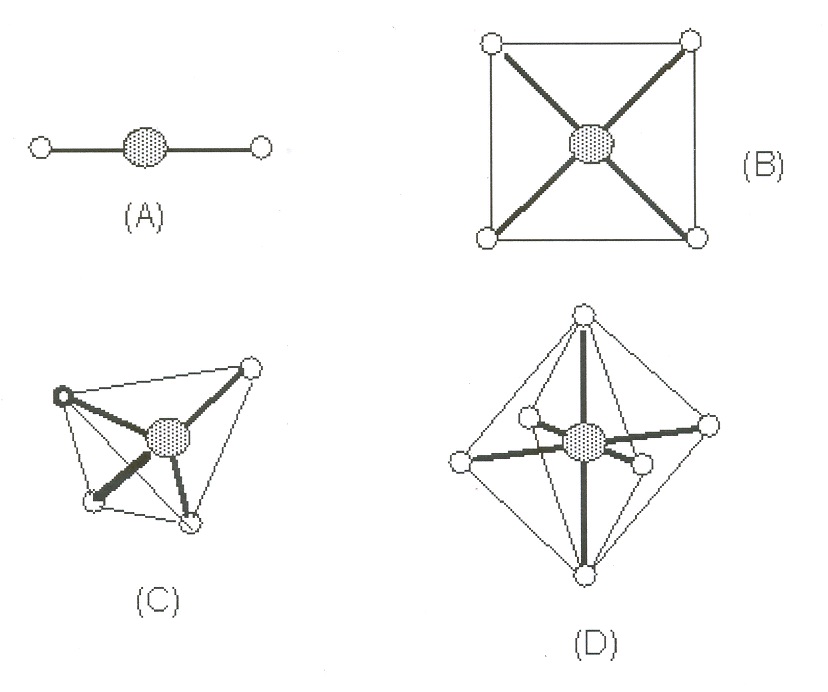









Stereochemistry
The most common coordination numbers (the number of individual ligands bound) are two, four,
and six, with geometries illustrated in Fig 1:
Figure 10.1.
Fig 1. Common geometries for complex ions. (A) linear, (B) square planar, (C) tetrahedral, and
(D) octahedral
Complexes of Cu(I), Ag(I), Au(I) and some of Hg(II) form linear structures (A) such as
,
, etc. Four-fold coordination (C) is not too common with transition metals, and the square
planar geometry (B) occurs in complexes of Pd(II), Pt(II), Ni(II), Cu(II), and Au(III). Six-fold coordination (D) is the most common and in fact the one we will study in this laboratory exercise.
A ligand that is capable of occupying only one position in the inner coordination sphere by
forming only one bond to the central atom is called a monodentate (“one tooth”) ligand. Examples are F−,
,
, H 2 O,
and
. If the ligand has two groups that are capable of bonding to
the central atom, it is called a bidentate ("two teeth") ligand, and so forth. An example of a bidentate ligand is ethylenediamine
, which is commonly abbreviated "en". Both
nitrogen atoms in "en" can bond to the central atom in a complex at the same time.
Complex ion salts with the same chemical formulas often behave differently because the same
number of atoms can be arranged into different forms called isomers. Hydrate isomerism is
illustrated by the following example: There are three distinct compounds with the formula
. One of these, violet in color, reacts immediately with
to precipitate all of the












chlorines as AgCl. The second is light green but only ⅔ of the chlorine is precipitated as AgCl.
The third compound is dark green and only ⅓ of the chlorine is precipitated as AgCl. The last
compound has only one reactive Cl, so apparently two chlorines in this compound are bonded
tightly to the Cr and are not available for reaction. We might thus write this compound as
, where the species within the brackets are regarded as ligands bonded fairly
strongly to the central chromium, and this species would behave as a single ion in solution. i.e., in aqueous solution,
The light green compound with two reactive chlorines is apparently
, while the
violet compound with three reactive chlorines is
.
Closely related to hydrate isomerism is ionization isomerism, where an ion takes the place of
water. Consider two different compounds with the formula
. One of these,
, appears red, whereas the other,
, appears violet.
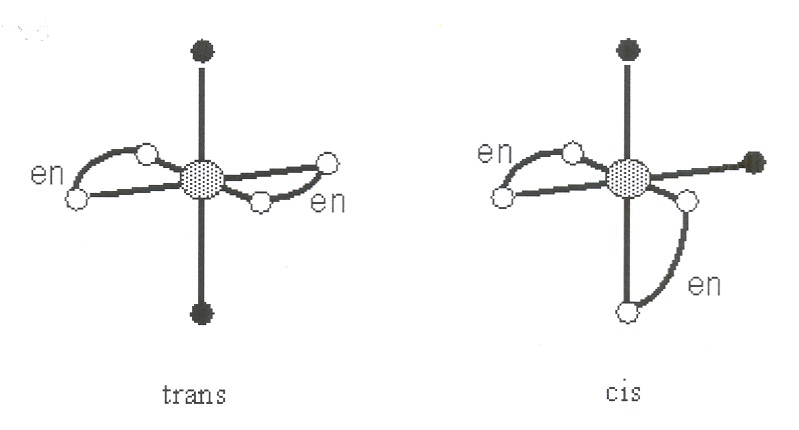
In addition to these coordination sphere isomers there are geometrical isomers, which have
coordination spheres of the same composition but different geometrical arrangement. Geometrical isomers are distinct compounds and can have different physical properties (although often not too different) such as color, crystal structure, melting point, and so on. For example, dichlorodiamine platinum (II) occurs in the square planar geometry (B) so the chlorine ligands can be either next to one another (cis) or opposite from one another (trans). The compound you will synthesize has an octahedral geometry with two (bidentate) "en" ligands, and two nitro
ligands. The
geometrical isomer you will make is the trans form, in which the
ligands are not adjacent to
one another. This difference between cis and trans octahedral isomers is shown in Fig 2.
Figure 10.2.
Fig 2. The trans and cis geometrical isomers for octahedral complexes with two bidentate (“en”) and monodentate
ligands specifically dinitrobis(ethylenediamine)Co(III). The two black
balls represent the
ligands and the two pairs of linked white balls represent the two
ethylenediamine ligands. Cis and trans describe the relationship (relative position) between the two
ligands.








In the procedure that follows we start with a cobalt solution made from the salt hexaquacobalt(II) nitrate,
. When this salt dissolves it ionizes to form two ions of
and one of
. We wish to prepare a Co(III) compound of ethylenediamine, so we must add
ethylenediamine (en) and oxidize the Co(II) to Co(III). Because Co(II) is more reactive than
Co(III), we allow it to react with (en) first, and then oxidize the resulting complex ion. In aqueous solution (en) reacts with water to produce
ions which can also bind to Co(II), so the pH is
adjusted close to 7 first by adding
. (Other acids would introduce new ligands to compete for
the Co.)
is added to provide the ligands that will be trans in the final compound. Lastly,
Co(II) is oxidized to Co(III) by bubbling oxygen through the solution.
Experimental Procedure
1. Use your 10 mL graduated cylinder to


































































