 HIMSS Stages
HIMSS Stages
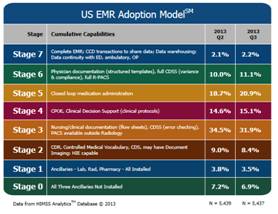
HIMSS Analytics website http://www.himssanalytics.org says
they support improved decision making for healthcare organizations, healthcare
IT companies and consulting firms by delivering high quality data and
analytical expertise.
They have created this EMR Adoption Model (there is another
model for Canadian hospitals and have recently released a version for the
Ambulatory setting). They think if the hospitals go through these 8 stages they
will have a “complete” EMR and participation from staff at their facilities and
be a completely paperless environment.
The following steps are taken from the whitepaper which is
located at: http://www.himssanalytics.org/docs/HA_EMRAM_Overview_ENG.pdf
and is each step to becoming a paperless environment.
Stage 0: The
organization has not installed all of the three key ancillary department
systems (laboratory, pharmacy, and radiology).
Stage 1: All
three major ancillary clinical systems are installed (i.e., pharmacy,
laboratory, and radiology).
Stage 2: Major
ancillary clinical systems feed data to a clinical data repository (CDR) that
provides physician access for reviewing all orders and results. The CDR
contains a controlled medical vocabulary, and the clinical decision support/rules
engine (CDS) for rudimentary conflict checking. Information from document
imaging systems may be linked to the CDR at this stage. The hospital may be
health information exchange (HIE) capable at this stage and can share whatever
information it has in the CDR with other patient care stakeholders.
Stage 3: Nursing/clinical
documentation (e.g. vital signs, flow sheets, nursing notes, eMAR is required
and is implemented and integrated with the CDR for at least one inpatient
service in the hospital; care plan charting is scored with extra points. The
Electronic Medication Administration Record application (EMAR) is implemented.
The first level of clinical decision support is implemented to conduct error
checking with order entry (i.e., drug/drug, drug/ food, drug/lab conflict
checking normally found in the pharmacy information system). Medical image access
from picture archive and communication systems (PACS) is available for access
by physicians outside the Radiology department via the organization’s intranet.
Stage 4: Computerized
Practitioner Order Entry (CPOE) for use by any clinician licensed to create
orders is added to the nursing and CDR environment along with the second level
of clinical decision support capabilities related to evidence based medicine
protocols. If one inpatient service area has implemented CPOE with physicians
entering orders and completed the previous stages, then this stage has been
achieved.
Stage 5: The
closed loop medication administration with bar coded unit dose medications environment
is fully implemented. The eMAR and bar coding or other auto identification
technology, such as radio frequency identification (RFID), are implemented and
integrated with CPOE and pharmacy to maximize point of care patient safety
processes for medication administration. The “five rights” of medication
administration are verified at the bedside with scanning of the bar code on the
unit does medication and the patient ID.
Stage 6: Full
physician documentation with structured templates and discrete data is
implemented for at least one inpatient care service area for progress notes,
consult notes, discharge summaries or problem list & diagnosis list
maintenance. Level three of clinical decision support provides guidance for all
clinician activities related to protocols and outcomes in the form of variance
and compliance alerts. A full complement of radiology PACS systems provides
medical images to physicians via an intranet and displaces all film-based
images. Cardiology PACS and document imaging are scored with extra points.
Stage 7: The
hospital no longer uses paper charts to deliver and manage patient care and has
a mixture of discrete data, document images, and medical images within its EMR
environment. Data warehousing is being used to analyze patterns of clinical
data to improve quality of care and patient safety and care delivery
efficiency. Clinical information can be readily shared via standardized
electronic transactions (i.e. CCD) with all entities that are authorized to
treat the patient, or a health information exchange (i.e., other non-associated
hospitals, ambulatory clinics, subacute environments, employers, payers and
patients in a data sharing environment). The hospital demonstrates summary data
continuity for all hospital services (e.g. inpatient, outpatient, ED, and with
any owned or managed ambulatory clinics).
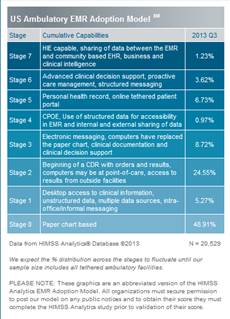
Here are the current
Ambulatory Stages:
 HIPAA Omnibus Rule
HIPAA Omnibus Rule
The government created the Health Insurance Portability and
Accountability Act of 1996 (HIPAA) which is governed by The U.S. Department of
Health and Human Services (HHS) Office for Civil Rights (www.hss.gov) and that website states it was
established “to strengthen the privacy and security protections for health
information”. Then they created the Omnibus rule which required providers to be
compliant by September 23, 2013 or face consequences (some changes allow you to
not be completed until September 23, 2014). The new Omnibus rule focused on
three areas:
• Privacy, Security, and Breach Notification policies and
procedures
You are not able to discriminate
based on GINA (Genetic Information
Nondiscrimination Act of 2008). This is now tied into HIPAA since genetic
information is part of health information. You are not allowed to use or
disclose genetic information for underwriting purposes. That leads us into the
changes on your Notice of Privacy Practices. Those must be updated and include
provisions that indicate:
§
The health plan will notify affected
participants if a breach of unsecured PHI occurs
§
The plan may not use or disclose PHI that is
genetic information for underwriting purposes, consistent with GINA
§
The plan will obtain an individual's
authorization before it uses PHI for marketing purposes, sells PHI, or uses or
discloses PHI for any purposes not described in this notice.
Patients have more individual
rights under the new law. They are able to request copies of their health
records in an electronic format (which is also a requirement of Meaningful Use
Stage 2). Also individuals who pay with cash can decide whether or not to allow
the provider to share information about their treatment with their health plan.
You are also limited on how the information is used and disclosed for marketing
and fundraising purposes. Patient health information cannot be sold without
their permission. Along with this, the patient also has the ability to deny you
the right to use their health information for research purposes. It does make
it easier for you to share immunization data with a child's school (you have
one year after September 23, 2013 to modify contracts with your business
associates to comply with this rule).
• Notice of Privacy Practices (NPP)
Notice of Privacy Practice that
most hospitals hand to patients and have displayed on their websites will need
to have some more clarification added. Once you make these revisions (this had
to be completed before September 23, 2013) you must post that changes have been
made and also alert patients on the change and how they can obtain a copy of
the changes.
• Business Associate (BA) Agreements
Business Associates of covered
entities are directly liable for compliance with the new regulations. This now
includes contractors and subcontractors since the largest majority of breaches
in the past have been attributed to business associates (according to Dolbey
almost 57% are from BA's). Noncompliance penalties have increased up to $1.5
million for each violation (and up to 10 years imprisonment). These penalties
are now tier based with increasing penalties based on the level and severity of
a violation. The term Business Associate used to mean anyone who performs or
assists in the performance of a function or activity involving the use or
disclosure of protected health information (PHI). Now it has expanded to
include persons who create, receive, maintain, or transmit PHI in connection
with performing a function or service for a covered entity, even if they do not
view the PHI. If you have an existing BAA and that agreement is not renewed or
amended from March 26, 2013-September 23, 2013 it is still compliant until it
is renewed or amended after September 23, 2013 or before September 23, 2014
(whichever occurs earliest). You must still document any risk assessment
performed, but now an impermissible acquisition, access, use or disclosure of
PHI is a presumed breach that must be reported. You must report the breach or
if it did not constitute a breach and document why it was not a breach. In
those cases you must do a risk assessment on these factors at the minimum:
1) What
was the nature and extent of the PHI involved (list the types of identifiers
and the likelihood of re-identification of this information)
2) Who
was the unauthorized user who accessed the data or to whom did they disclose it
3) Was
the PHI acquired or viewed
4) What
extent is the risk to the PHI mitigated (is it a risk of financial, reputation,
or other harm)
Some
important revisions that need to be included in your Business Associates
Agreement (BAA) include:
- Comply with the
applicable provisions of the Privacy Rule;
- Comply with the
Security Rule regarding electronic PHI;
- If the Business
Associate (BA) enters into an agreement with any subcontractors, then
the contractor (BA) must provide assurance that their subcontractor will
appropriately safeguard the PHI and agree to the same protections and
restrictions as the current agreement between the covered entity (MMCWM)
and business associate; and
- Report a breach of
unsecured PHI to the covered entity
For all current Business Associate
Agreements that you have in place, you will need to review and determine if any
of those vendors have not identified their subcontractors and ensure they have
Business Associate Agreements in place. You will want to ensure this is in
place so that the liability falls to them and not to your organization.
If you want to read the entire Final Rule in the Federal
Register, it is located at http://www.gpo.gov/fdsys/pkg/FR-2013-01-25/pdf/2013-01073.pdf








