Chapter 2
It Really Works But How?
Hydrogen peroxide, which properly should be called "hydrogen dioxide, is a colorless (blue in thick layers), odorless liquid. Its melting point is minus two degrees Celsius, and its boiling point is 152 degrees Celsius. It is soluble in water at all concentrations and it is usually encountered as a dilute solution of three percent. Hydrogen peroxide is used (1) as a bleaching agent; (2) as an antiseptic and disinfectant; (3) as an oxidizing agent, and (4) as an oxidizer in rocket motors for small rockets.
Hydrogen peroxide solutions dismutate (i.e., break down) slowly when undisturbed at about the rate of one percent per month. Contrary to popular belief, hydrogen peroxide is not unstable, and even when heated, it will break down very slowly. If this dismutation reaction is rapidly increased in the presence of contaminants such as dust, metal, or glass, it may be quite explosive. Cold retards the dismutation and solutions may be refrigerated or stored at temperatures below zero degrees Celsius. Hydrogen peroxide occurs only in traces in nature, mostly in rain and snow. It has not yet been detected in interstellar space.1
Early studies on H2O2 infusions predicted that its half-life is less than one-tenth of a second. However, more recent studies by MacNaughton calculated that the halflife of peroxide ranges from three-quarters of a second to two seconds and is dependent upon the rate of mixing in the blood.
All species of animals do not react the same to peroxide because there are species differences in catalase enzyme content between man and animal. So the results from many animal models will not correlate with what happens in man and, therefore, are not applicable to man. Dogs and chickens, for instance, have very low catalase levels, and so they have poor tolerance to H2O2 . In fact, you can kill them with hydrogen peroxide. They will develop pulmonary edema and methemoglobinemia. However, in man, catalase is abundant in both the plasma and red cells and is significantly elevated in diseases such as rheumatoid arthritis.2 It can be used in dogs, as you will soon see from the reports in this chapter. You just have to be careful.
Hydrogen peroxide initially reacts with catalase in the plasma and the white blood cells. Later, it penetrates the cell membrane of erythrocytes (red blood cells), where it reacts with catalase within the cell, and additional oxygen is then released.
Some of the biological killing activities of hydrogen peroxide may be attributed to interferon. Production of interferon by human killer cells and monocytes is stimulated by hydrogen peroxide.
Studies have been done comparing hyperbaric oxygen (giving the patient oxygen under pressure in a high pressure tank) and intravenous hydrogen peroxide to compare the level of the oxygen content in tissues.3 These researchers found that the tissue oxygen levels with intravenous hydrogen peroxide paralleled the increase in oxygen found with hyperbaric oxygen pressure treatment. This is a very important finding, because hyperbaric oxygen treatment is expensive, does have some risks, is rather cumbersome, and is generally not available.
Conversely, intravenous hydrogen peroxide is more readily available, is relatively cheap, safe, and quite effective. Also of great importance, Dr. Charles Farr found that increased oxvgen content of tissues often was not recorded until 40 to 45 minutes after the beginning of the peroxide injection. This probably explains why some investigators did not find a rise in the tissue oxygen pressure, because they measured it too soon. These investigators speculated that any increased venous oxygen saturation in tissues would be lost by diffusion of oxygen in the pulmonary capillary bed of the lungs. But Farr found this to be in error.4
If you're not interested in the physiological reasons why Dr. Farr found previous assumptions to be in error concerning the amount of oxygen absorbed through intravenous hydrogen peroxide, we suggest you skip the following paragraph:
If oxygen, released from intravenous H2O2 , diffuses from the pulmonary capillary bed into the alveolar space, alveolar pO2 will rapidly increase and pulmonary capillary blood pO2 will decrease. Diffusion into the alveoli will occur more rapidly than the alveolar loss of oxygen to respiratory exchange. Inspired oxygen added to the oxygen diffused into the alveoli from the pulmonary capillary at the arterial end would increase the alveolar pO2 greater than the blood pO2 at the venous end of the capillary. The increased pO2 in the alveolus would cause the oxygen to rapidly diffuse back into the pulmonary capillary at the venous side and go back into systemic circulation. This postulate was confirmed by studies of pulmonary oxygen uptake to determine metabolic rate in subjects receiving various concentrations of intravenous hydrogen peroxide.5
Welcome back.
We have noted, as has Dr. Farr, that blood specimens taken during and after hydrogen peroxide infusions show a color change consistent with an increase in oxygen content of the blood after the infusion. We sent a sample to the laboratory, and, although it was a venous specimen, the lab reported back that it must have been an arterial sample because of the high oxygen color of the blood.
After an hour of infusion of hydrogen peroxide, a 210 percent decrease will be noted in many blood constituents—such as sodium, potassium, chloride, phosphorus, etc. Twenty-four hours later, all of these constituents of the blood will have returned to normal pre-infusion levels.
* * *
The clinical benefit of oxygen saturation of tissue fluid from the oxygen produced by hydrogen peroxide may be of secondary importance. Very little peroxide is used in the treatment and, hence, very little oxygen is actually produced. Hydrogen peroxide is a powerful oxidizer, however, and will oxidize toxic and nontoxic substances alike, which is completely separate from its role as an oxygen contributor. Farr describes the biologic effects observed from the intravenous administration of H2O2 as "oxidative detoxification." The oxidative benefits may include the oxidation of lipid material in the vessel wall to reverse atherosclerosis.6 There are many other physiological benefits to oxidative detoxification, but it is too technical for this book. However, if you wish to investigate further, we recommend the article by Weiss, in the Journal of Clinical Investigation (1981;68:714-721). Weiss discusses things that I'm sure you remember from your high school biology course, such as aggregated immunoglobins, immune complexes, and bacterial peptides.
Peroxide is the ammunition of your killer cells. Your body's elite corps of bacterial assassins, called polymorphonuclear leukocytes (PMN's), engulf bacteria then kill them with the "respiratory burst." The cell combines oxygen and water, making H2O2 . That's the respiratory burst. The H2O2 then zaps the bacteria.
Those PMN's are really smart. First they identify the invader. (How do they do that, with no eyes and no brain?) Then they move to the attack. (No legs, either.) On contact, they gobble the bacteria and zap it with H2O2 . Amazing!
If your white cells didn't produce H2O2 , the respiratory burst would not be possible, and bacteria would have taken over the world a long time ago. So hydrogen peroxide has been promoted from an ordinary mouthwash to one of life's most important bodyguards. (The Bird Man of Alcatraz knew what he was talking about. He was a convict who had a special love for birds. He would treat a sick bird, who happened onto the island, with hydrogen peroxide and had quite phenomenal results in curing his little patients. Hence his nickname, The Bird Man of Alcatraz.)
And speaking of mouthwash, don't throw that bottle of hydrogen peroxide sitting in your medicine cabinet away. It's still better than Scope, Lavoris, Cepacol, or any other of those red and green liqueurs peddled on TV. It kills bacteria, retards gingivitis, and reduces plaque formation. It costs about one-tenth what you'd pay for those dessert drinks. Studies have shown that Legionnaire's disease,7 syphilis,8 yeast (candida), viruses, and even parasites will respond to hydrogen peroxide.
Hydrogen peroxide seems to be the all-purpose executioner. The Middlesex Hospital Medical School in London experimented with H2O2 in the treatment of malaria, which is a parasite rather than a bacterium. They found it to be effective.9
Hydrogen peroxide is truly the wonder molecule. The cells in your body that fight infection, called granulocytes, produce H2O2 as a first line of defense against every single type of invading organism—parasites, viruses, bacteria, and yeast. No other chemical compound comes even close to H2O2 in its importance to life on this earth. H2O2 is involved in all of life's vital processes. Protein, carbohydrate and fat metabolism, vitamin and mineral metabolism, immunity, and anything else involving life's functions require the presence of this amazing molecule.
There are over 6,100 articles in the scientific literature dating from 1920 on the scientific applications of hydrogen peroxide. It seems inconceivable that the astounding medical cures reported in science journals over the past 75 years could have been ignored. The reasons for this scientific blindness will become apparent to you as the peroxide story unfolds.
In some mysterious way not yet identified, H2O2 is involved in phagocytosis, the process by which some of your blood cells eat enemy bacteria. H2O2 also acts like insulin, in that it aids the transport of sugar through the body.
Hydrogen peroxide may be just as important, or more important, than thyroid for heat generation. As you know, your car won't run properly if it is cold. Neither will your body. H2O2 , in the presence of coenzyme-Q10, creates "intracellular thermogenesis," a warming of your cells which is absolutely essential to life. As one researcher put it, the new information on H2O2 affords the conceptual basis for a revolution in our thinking about many of life's vital processes.
It's amazing how medicine has largely ignored this well-researched and unique therapy. But what's new? Some doctors still don't wash their hands between patients, although Dr. Ignatz Semilweise proved a hundred years ago that doctors were the main cause of the spread of infection in hospitals because of their contaminated hands. Nothing changes. Peroxide therapy will continue to be resisted and ridiculed by American doctors.
Most of the work at Baylor University, which we will discuss in the next chapter, was done by dripping H2O2 into an artery. It really isn't necessary to use the more difficult arterial route for peroxide therapy.
One of our colleagues measured a pulmonary (lung) patient's arterial pO2 level (the measure of oxygen content), before and after hydrogen peroxide therapy. After the infusion of hydrogen peroxide, this patient's oxygen content went from 60 to 80, which is a marked improvement. You can, in fact, just look at venous blood when it's drawn from the patient following a peroxide treatment and see a marked difference in the color. It assumes the color of arterial blood, which contains more oxygen than venous blood.
Hydrogen peroxide is also necessary for the manufacture of hormone-like substances called prostaglandins. Also, hydrogen peroxide produced by ascorbic acid (vitamin C) has been shown to induce prostaglandin synthesis. This would suggest that the beneficial clinical effects observed with the use of Vitamin C in inflammatory reactions, and its protective action against infections, result from the generation of hydrogen peroxide, which in turn induces the production of prostaglandins.
Doctors at the Boston University Medical Center10 compared the effectiveness of hyperbaric oxygen and H2O2 in their ability to oxygenate tissues. They put rabbits in pressure chambers and pumped in oxygen. They compared the level of tissue oxygen with the level found when H2O2 was given in an artery or a vein.
I'd better explain the difference between an artery and a vein—many people don't know. If you do know (or don't care), then skip the next paragraph.
The veins are the blood vessels that you see on the back of your hand, the arms and feet. You can't see arteries. The veins return the blood to the heart from the far reaches of the body. The arteries deliver the blood back to the body from the heart after it has gone through the lungs to pick up oxygen (see below).
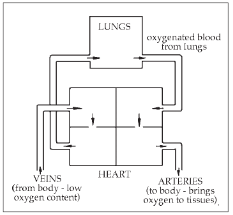
The doctors found that if they gave H2O2 into an artery, it was just as effective at raising tissue oxygen levels as the hyperbaric method. But given in the vein, there was no rise in oxygen levels of the tissues.
This difference is important because it is easy to give medication in a vein, but not so easy to give it through an artery. There are many reasons for this. The main one being that arteries are not as accessible as veins. It should be noted, however, that current clinical trials refute the claim that H2O2 doesn't work when given in the vein.




