CHAPTER 5
MEDICAL WASTE
5–1. Classification
a. Regulated medical waste (RMW) is a term that describes wastes generated by medical, veterinary, and dental treatment facilities in the diagnosis, treatment, research, or immunization of human beings or animals which are potentially capable of causing disease, and may pose a risk to either individuals or community health if not handled or treated properly. These types of wastes are defined in United States Army Medical Command (MEDCOM) Regulation 40-35 and include: cultures and stocks of infectious agents; pathological waste (tissues, organs, body parts, teeth); human blood and blood products; contaminated animal carcasses, body parts, and bedding used in animal research; isolation waste from patient rooms; sharps (syringes, scalpels, blades); and human body fluids (semen, vaginal secretions, cerebrospinal fluids, pleural fluids).
b. There may be diseases unique to a specific theater. The theater surgeon should designate whether or not nonbloody wastes from these diseases require segregation and management as RMW. The decision is based on the nature of the disease, prevalence, the method of transmission, and other risks.
c. Whole bodies are not considered RMW. Quartermaster units will manage human bodies according to Joint Publication (JP) 4-06.
d. Animal body parts, carcasses, and bedding (not contaminated by medical research) are not considered RMW, and may be incinerated or landfilled.
e. Personnel handling blood-soaked clothing or personal equipment (such as body armor) should adhere to the handling guidance provided in paragraph 5–2a below. To render items non-infectious, wash blood-soaked items with soap and hot water. Adhere to the cleaning guidance provided on clothing/equipment labels, or consult with Quartermaster personnel for detailed laundering instructions. Equipment that remains stained after laundering should be returned through supply channels for either turn-in or exchange. Logistics personnel will evaluate item serviceability and make the final decision regarding disposition of government-issued clothing and equipment.
5–2. Handling
a. Use standard precautions when handling wastes generated as a result of treating patients or animals. Personal protective equipment includes protective gloves (disposable latex, butyl rubber, or other types impermeable to blood), masks/safety goggles/safety glasses, or other equipment that will prevent personnel from contracting communicable illnesses from patients or their wastes. Any exposed skin should be washed with soap and water.
b. Personnel should wear both skin protection and respiratory protection when burning or incinerating medical waste, and should avoid standing in the resulting smoke plume. BURNING RMW OR ANY OTHER WASTE IS NOT AUTHORIZED FOR CONUS FIELD TRAINING EXERCISES. An air-purifying respirator (cartridge or canister) with a high-efficiency particulate air (HEPA) filter is recommended (see figure 5–1). Commercial respirators approved by the National Institute for Occupational Safety and Health with a P100 or N100 rating are preferred. The M40 protective mask should only be used until commercial respirators are obtained. Paper surgical masks do not protect from hazards inherent in the burning of waste and should not be substituted for an air-purifying respirator. Respiratory protection is only needed for those personnel remaining in the immediate vicinity of the burning process. Personnel tasked to incinerate medical waste must be medically cleared to wear a respirator, properly fit-tested on an approved respirator, and enrolled in a medical surveillance program.

c. Consider vaccinating all employees that handle RMW against the Hepatitis B virus. This immunization is mandatory for U.S. medical personnel.
5–3. Collection, segregation, and storage
a. Contact the supporting Medical Logistics unit or Class VIII manager for medical waste storage containers. Collect RMW at the point of generation in red bags (or other color specified for the theater). Sharps will be collected in puncture-resistant, leak-resistant, and uniquely colored or marked containers. If proper sharps containers are not available, use any rigid plastic or metal containers (such as coffee cans or plastic drink bottles) for collection. These nonstandard containers should be placed into red bags or proper sharps containers as soon as possible for disposal.
b. All bags or receptacles used to segregate, transport, or store RMW will be clearly marked with the universal biohazard symbol and the word “BIOHAZARD” in English (see figure 5–2) and any other language suitable for the region.

c. Never mix RMW with regular trash or HW, unless required for the burning process. Medical personnel should also take care to ensure clothing and bandages placed into red bags do not contain ammunition or other unexploded ordnance.
d. Store RMW in secure, ventilated areas that offer protection from the sun, rain, scavengers, and pests. Collection in a covered cargo trailer facilitates the transport of the waste from the medical facility.
e. Medical waste (other than sharps containers) should not be stored above 40°F (4.4°C) for longer than 5 days.
5–4. Transporting regulated medical waste in the field environment
Regulated medical waste is considered an HM for transportation purposes and must comply with the requirements of 49 CFR 100-185 and DOD 4500.9-R. Additionally, RMW transported by military aircraft must comply with the AFMAN 24-204(I).
a. Transport RMW in military, government, or contractor vehicles. Use of privately owned vehicles is not authorized.
b. The RMW must be secured to prevent excessive movement and will not be transported alongside items intended for consumption.
c. A spill kit must be readily available to decontaminate any surfaces in the event of a leak or spill and shall include: appropriate PPE, a disinfectant, absorbent material, and equipment used to gather spill residues. The kit may be assembled at the local level or purchased commercially.
d. If RMW must be transported across public roads, the driver must receive training according to 49 CFR 177.
e. Vehicles used to transport RMW must be cleaned and disinfected prior to use for any other purpose.
5–5. Treatment and disposal
On installations in the United States and overseas, commercial contractors will be used for RMW disposal (DLA/DRMS does not manage medical waste). All RMW generated during field exercises should be backhauled to garrison. During contingency operations, RMW may be incinerated, burned, sterilized, or buried according to guidance provided in the combatant command’s operations order. Incineration and burn activities should be conducted as far downwind as possible (at least 450 feet) from troop locations and living areas.
a. Incineration. Use of a commercial incinerator capable of subjecting the waste to a minimum burn temperature of 1500°F (816°C) for at least 1 hour is the preferred method of destruction. Incinerator operators must be trained on proper operating and maintenance procedures, safety measures (to include PPE use), emergency response, and local environmental requirements. Incinerator bottom ash and air pollution control ash (if applicable) should be tested for HW properties prior to disposal in a solid waste landfill. Aerosol cans, gas cylinders, and batteries should never be incinerated. Seek approval from the local commander prior to operating field-expedient devices such as the inclined-plane incinerator with vapor burner (described in FM 4-25.12).
b. Burning in barrels. Burning medical waste in barrels or pits is permissible provided these burns are approved by appropriate command personnel and local officials, and conform to regulatory policies for the specified region. Whenever possible, avoid burning when wind and other conditions could cause the resulting smoke to blow in the direction of personnel at the base camp. Only personnel involved in the actual burning need to wear respiratory protection as described in paragraph 5–2b. To ignite the burn, mix one part gasoline with five parts JP-8. Use a stick or pole to light the fuel from a distance of at least 3 feet. Mixing medical waste with regular solid waste (approximately 50/50 mixture) will help ensure the hottest and cleanest burn possible. The remaining ash may be buried in a solid waste landfill. Aerosol cans, gas cylinders, and batteries should never be burned.
c. Sterilization. Steam sterilization is another alternative to treatment of medical waste. Ensure the waste is secured in autoclave bags (regular plastic bags may melt) prior to placement in the sterilizer. Autoclave indicator tape, if available, will demonstrate when sterilization is complete. Guidelines for minimum operational temperatures and detention times are: 250°F for 90 minutes at 15 pounds per square inch (psi) gauge pressure, 272°F for 45 minutes at 27 psi gauge pressure, or 320°F for 16 minutes at 80 psi gauge pressure. After the RMW is sterilized and cooled, the waste may be managed as general trash. Ensure care is taken when handling the waste to minimize needle sticks. STERILIZERS USED TO AUTOCLAVE MEDICAL WASTE MUST NEVER BE USED TO STERILIZE OTHER MEDICAL ITEMS (such as medical instruments or dressings). Permanently and indelibly mark medical waste incinerators as “For Medical Waste Only—Do Not Use for Sterilization” or words to that effect. Have a contingency plan in place to manage waste that was intended for sterilization if the steam sterilizer becomes nonfunctional.
d. Retrograding. Retrograding waste back to the rear where facilities are available may be feasible if burning, incineration, or sterilization is not possible. International agreements govern the retrograde of medical waste, and any such movement must be coordinated through the combatant command.
e. Burial. The last resort is burying untreated medical waste in a sanitary landfill. This method should be employed only during contingency operations in areas with low water tables. Care must be taken to bury RMW below the scavenger depth of 8 feet. A layer of lime may be placed over the waste prior to burial to accelerate decomposition and provide a measure of chemical disinfection. Because the Army will most likely have to retrieve this waste later, medical waste burial sites must be marked and grid locations reported through the chain of command.
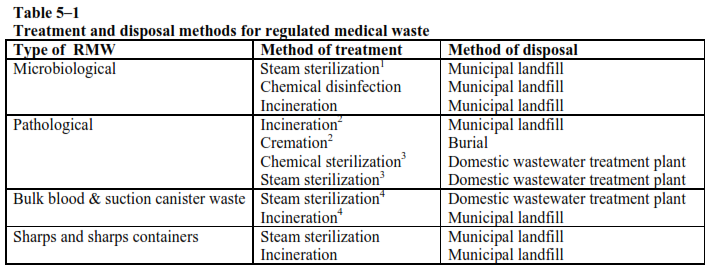
f. Alternative technologies. Alternative technologies may also be used to treat and dispose of RMW. If connected to a domestic wastewater treatment plant, bulk blood or blood products may be poured into clinical sinks. See table 5–1 for more information.
Notes
1 Preferred method for cultures and stocks because they can be treated at point of generation.
2 Anatomical pathology waste (that is, large body parts) must be treated either by incineration or cremation prior to disposal.
3 This only applies to placentas, small organs and small body parts that may be steam sterilized or chemically sterilized, ground, and discharged to a domestic wastewater treatment plant.
4 Bulk blood or suction canister waste known to be infectious must be treated by incineration or steam sterilization before disposal.
5–6. Disposal of drugs
Consult the most recent edition of Supply Bulletin 8-75-11, Medical Materiel Quality Control (MMQC) messages, and the MIDI database for guidance on pharmaceutical disposal. Drugs that cannot be returned to the manufacturer, and that meet the criteria for HW, must be managed according to chapter 4 of this TB MED. If a waste determination cannot be made with local knowledge, contact the USACHPPM at 1–800–276–MIDI.
a. Immunizations. The United States Army Medical Materiel Agency frequently publishes disposal instructions for common immunizations in MMQC messages. In the absence of more specific guidance, collect discarded vaccines and other immunizations in sharps containers and incinerate.
b. Controlled substances. Special care must be taken to maintain accountability of controlled substances during disposal. Disposal guidance for these items is provided in Defense Logistics Agency Regulation (DLAR) 4145.11. Many controlled substances, such as morphine, may be safely disposed by flushing into a sanitary sewer.
c. Chemotherapy wastes. Chemotherapy (also known as cytotoxic or antineoplastic) wastes must be incinerated in special high-temperature incinerators capable of achieving HW treatment standards. These wastes must never be buried.
d. MNBCDM. Follow MIDI guidance for the disposal of MNBCDM waste. Many of these items can be managed as RMW or solid waste. Some MNBCDM wastes that require special disposal considerations include: atropine (should be incinerated to safely destroy auto-injectors), diazepam (controlled substance), and pyridostigmine bromide tablets (should be managed as a listed HW due to acute toxicity). Figure 5–3 displays a common source of MNBCDM waste.









