FITNESS COSTS OF SAR
It has often been suggested that disease resistance is associated with fitness costs and that plants have evolved inducible defense mechanisms because it is too costly to have defense responses switched on all the time. The phenotypes of many mutants that show constitutive PR gene expression, accumulation of SA, and resistance to pathogens support this idea. These mutants often have reduced plant size, loss of apical dominance, curly leaves, and decreased fertility, all traits associated with decreased plant fitness. Consistent with this view, overexpression of NPR1 in rice triggers lesion development and chlorosis under certain environmental conditions, correlating with expression of defense genes. Although only a few studies have specifically addressed the fitness costs of SAR, they all conclude that constitutive expression of SAR in uninfected plants is detrimental.
The SA analogue BTH, which has been developed commercially as a plant activator, induces SAR in wheat and confers systemic protection against powdery mildew. Researchers have therefore studied the fitness costs associated with the use of BTH in the absence of pathogens. Plants grown hydroponically in the absence of pathogens or in the field showed a reduction in biomass and the number of ears and grains if treated with BTH. These effects were stronger when combined with limited nitrogen availability. Similarly, treatment of Arabidopsis with SA decreases seed yield. These experiments, although not representative of natural conditions, are useful when considering the costs and benefits of treating crop plants with such compounds.
An alternative to exogenous chemical treatment is to examine the fitness of mutants with constitutive SAR or mutants impaired in SAR. Two studies have shown that gain-of-resistance mutants such as cpr1, cpr5, cpr6, and cep1 [constitutive expression of PR-1; have decreased fitness, demonstrated by low seed yield and small rosette diameter. The cost of resistance observed here could be due to the allocation of the plant's resources to constitutive PR protein production. As well as a cost of constitutive SAR, there is a fitness cost associated with the inability to induce SAR. One study showed that nahG and nim1plants grown in a growth chamber have high seed production in the absence of SAR induction. However, in a field experiment, the npr1 mutant and the NPR1-Ltransgenic line, in which NPR1 is silenced, showed a reduction of fitness. Although there was no visible sign of disease on these plants, they might have had a low level of infection that was detrimental to plant growth. It is also possible that soil bacteria could have induced ISR in the wild-type plants, but not in npr1 or the NPR1-L line, to protect them from microbial pathogens. This study shows that SAR has fitness benefits and fitness costs consistent with the inducible nature of this defense response. Such results have important implications for development of crop protection strategies through manipulation of SAR.
Another important factor in helping sports turf acquire systemic acquired resistance(SAR) is the prevention of oxidative stress(free radical) damage.
Traditionally, reactive oxygen intermediates (ROIs) were considered to be toxic by-products of aerobic metabolism, which were disposed of using antioxidants. However, in recent years, it has become apparent that turf plants actively produce ROIs as signaling molecules to control processes such as programmed cell death, abiotic stress responses, pathogen defense and systemic signaling. Recent advances including microarray studies and the development of mutants with altered ROI-scavenging mechanisms provide new insights into how the steady-state level of ROIs are controlled in cells. In addition, key steps of the signal transduction pathway that senses ROIs in plants have been identified. These raise several intriguing questions about the relationships between ROI signaling, ROI stress and production and scavenging of ROIs in the different cellular compartments.
Oxidative Stress and the Role of Reactive Oxygen Species
The field of redox biology has recently witnessed a dramatic reappraisal of the importance of ROS. Due to the reactivity of ROS and because they are unavoidable by-products of oxygenic photosynthesis, only the more negative aspects of ROS generation are often considered in relation to observations. Thus, light-driven ROS production is generally described as harmful because it has the potential to cause irreversible damage to photosynthetic components. It is generally often suggested that ROS production should be minimized at all costs. However, despite their potential for causing harmful oxidations, it is now well established that ROS are also powerful signaling molecules that are involved in the control of turf plant growth and development as well as priming responses to stress stimuli. In many studies involving photosynthetic ROS generation, the signaling function of these powerful metabolites is largely ignored, and interpretations are all too frequently based solely on the notion that ROS exert their principal effects through chemical toxicity and their abilities to cause damage. Within this context, the term “oxidative stress” has become largely synonymous with “oxidative damage” to cellular components, particularly in situations where oxidative inactivation exceeds that of repair or replacement. Furthermore, it is often suggested that the accumulation of damaged cellular components and associated loss of function leads to cell death, but the mechanisms that cause cell death in this case are generally vague or undefined. Relatively few studies to date have considered photosynthetic ROS generation in the context of the light-driven production of powerful signaling molecules, whose abundance provides essential information to the cell concerning imbalances between energy-generating and energy-utilizing processes in the PET system. Within this context, increased ROS production leading to enhanced oxidation in high light may be considered to be a powerful signal that not only decreases PSII(Photosystem II) activity but also stimulates gene expression, particularly with regard to acclimation and defense gene. While concepts of ROS function in signaling or damage in photosynthesis are largely irrelevant to the description of the basic biochemical mechanisms by which ROS oxidize cellular components, the philosophical choice between “damage” and “signaling” in data analysis remains crucial to the evaluation of the physiological significance of these mechanisms.
Oxygen Production and the Regulation of Photosynthesis
Oxygenic photosynthesis is a dynamic and flexible process that powers life on earth, in which water oxidation on the lumen side of PSII is an indispensable step.
The light-driven PET system drives electrons from water through to NADP, generating the proton gradient that facilitates ATP synthesis. Intriguingly, a key feature of PSII is its vulnerability to light-induced damage, which is considered to be a consequence of the production of singlet oxygen in the PSII reaction center, leading to irreversible oxidation of the D1 protein. This sensitivity means that the PSII reaction center has to be rebuilt about once every 30 min even under relatively low irradiances. The damage and repair process thus occurs under all light intensities. A limitation of the PET system only occurs when the rate of damage exceeds that of repair, and this makes a major contribution to the processes called photoinhibition. In many circumstances where the rate of damage is fast, such as at high light intensities, the rate of repair is also rapid, so a high level of PSII activity can be maintained.
It is widely accepted that efficient regulation of PET serves to minimize the production of singlet oxygen at PSII as well as the generation of superoxide and hydrogen peroxide (H2O2), which occurs predominantly on the reducing side of PSI .Within this context, reversible decreases in the efficiencies of both photosystems are intrinsic to the regulation of light use by photosynthesis .Inherent limitations on the capacity for electron transport through the cytochrome b6/f complex favor over-reduction of PSII, which exacerbates PSII turnover. Of the strategies that can be employed to protect PSII under conditions of limiting PET capacity, the most important is non-photochemical quenching (NPQ), which dissipates the excitation energy of chlorophyll a molecules safely as heat.
The majority of electron flow follows a linear, noncyclic route from water through PSII, the cytochrome b6/f complex and PSI to NADP, leading to the generation of both NADPH and ATP. However, the operation of a cyclic electron flow pathway around PSI provides a mechanism whereby ATP production can be increased relative to NADPH. The operation of cyclic electron flow is also considered to prevent over-reduction of the acceptor side of PSI. This is important because superoxide and H2O2 are generated by PET components on the acceptor side of PSI. Cyclic electron flow may also help to limit singlet oxygen production at PSII because it enhances protonation in the lumen, which triggers protective NPQ mechanisms. The exact contribution of the linear and cyclic pathways to overall electron flow depends on cell type and environmental conditions. However, in C3 leaves under optimal growth conditions, the major function of cyclic electron flow around PSI is considered to be the augmentation of ATP production relative to NADPH in order to balance the energy budget of the chloroplast.
While many fundamental questions remain concerning the components involved in cyclic electron flow and the extent to which this pathway operates in C3 turf plants, such as Kentucky Bluegrass, perennial ryegrass, fescue, creeping bentgrass, there is a general consensus of opinion that cyclic electron flow is an essential component of the repertoire of chloroplast mechanisms that serve to coordinate energy metabolism and balance redox status. A further mechanism of regulation for photosynthesis that prevents over-reduction of the PSI acceptor side and the chloroplast stroma is the “malate valve” system, which transfers reducing equivalents to the cytosol. This pathway, which is activated by the thioredoxin (TRX)-regulated activation of chloroplastic NADP-dependent malate dehydrogenase, is an essential component for the stromal redox homeostasis network because it allows the export of excess reducing power and thus relieves electron pressure in the chloroplast. In this way, the regulation of metabolite distribution can be used to balance cellular redox status in order to achieve metabolic and photosynthetic control. However, to date, little attention has been paid to how the regulated distribution of other metabolites, particularly antioxidants, may alter the stromal redox homeostasis network and affect the regulation of photosynthesis.
THE ANTIOXIDANT NETWORK OF CHLOROPLASTS
Photosynthesis is an important source of cellular oxidants in the turf grass system. Even under optimal conditions, the calculated rate of H2O2 formation by the PET chain in the chloroplasts during photosynthesis in C3 turf leaves is nearly as high (4 μmol m–2 s–1) as the amount produced in the peroxisomes as a result of the glycolate oxidation in the photorespiratory pathway. While it is often stated that the amount of H2O2 in chloroplasts can increase by several orders of magnitude during stress, such notions must be viewed with caution because of the inherent complexities of obtaining accurate measurements of H2O2 contents in intact tissues and isolated organelles. Current methods do not allow accurate estimations of the H2O2 concentration of the stroma under either optimal or stress conditions. Regardless of any uncertainties about absolute values for H2O2 levels in photosynthetic tissues, it has long been recognized that H2O2 is a potent inhibitor of photosynthesis, because even at low concentrations it can inhibit CO2 fixation. The reduction of ground-state molecular oxygen to superoxide on the acceptor side of PSI in the Mehler reaction is the first step of a series of reactions that together has been called “the water-water cycle”. This incorporates superoxide dismutase (SOD), ascorbate peroxidase (APX), and the AsA-GSH cycle in a mechanism that builds up the trans- thylakoid proton gradient and facilitates ATP formation at the expense of NADPH and reductant APX utilizes AsA as a specific electron donor to reduce H2O2 to water with the concomitant generation of monodehydroascorbate (MDA), a univalent oxidant of AsA. While MDA is spontaneously converted to AsA and dehydroascorbate (DHA), it is also rapidly reduced to AsA by the action of a NADPH-dependent MDA reductase. DHA reductase (DHAR) utilizes GSH to reduce DHA and thereby regenerate AsA. GSH is then regenerated from oxidized glutathione, also called glutathione disulfide (GSSG), by the action of glutathione reductase (GR) using NADPH. While different APX and SOD isoforms are located in the stroma and thylakoid membrane, the chloroplastic GR and DHAR enzymes are localized in the stroma. A characteristic property of APX, particularly the chloroplastic APX forms, is their susceptibility to oxidative inactivation in the absence of AsA. Under low AsA concentrations, the activity of chloroplastic APXs is rapidly lost in the presence of H2O2, with a half-inactivation time of less than 30 s. In contrast, the cytosolic and peroxisomal APXs only lose activity after more than 1 h. The chloroplastic APXs are the primary targets for inactivation if chloroplast AsA accumulation is impaired. Depletion of chloroplast AsA and inactivation of chloroplast APXs, therefore, have been considered as limitations of photosynthetic efficiency in stress conditions and thus potential targets for improvement.
In addition to the AsA-GSH cycle, other proteins that are important in chloroplast ROS detoxification are peroxiredoxin (PRX; particularly 2-CysPrx and PrxQ), glutathione peroxidase (GPX), sulfiredoxin, and cyclophilin, which function together with TRX and TRX-like proteins in the chloroplasts. The thiol-based catalytic mechanism used by PRX to reduce H2O2consists of a peroxidative reduction, followed by regeneration that can involve a variety of electron donors such as TRX, glutaredoxin, cyclophilins, GSH, and AsA. GPXs can use both GSH and TRX as reducing substrates, and they can detoxify lipid peroxides as well as H2O2. Because TRX is a more efficient substrate than GSH and their high rates of TRX-dependent peroxidase activity, plant GPXs have been assigned to the PRX protein family. In the GPX system, the regeneration of reduced TRX is linked to the PET chain through either ferredoxin-TRX reductases or NADPH-dependent TRX reductases. While the catalytic rates of plant GPXs and their affinities for H2O2 are rather low compared with APX, the PRX and GPX pathways provide an alternative pathway to the water-water cycle in the light particularly if the AsA-GSH cycle is impaired. However, lipid peroxides are also efficient substrates for the chloroplast GPXs. The detoxification of lipid peroxides, which exacerbate the lipid peroxidation cascade reactions, may be as equally important as H2O2 detoxification in terms of maintaining optimal photosynthetic functions in the light. AsA-GSH cycle and the PRX-dependent detoxification pathways may be equally important in vivo and serve interfacing functions. Our view is that the two detoxification pathways are tailored to suit specific niches in defense metabolism and that their relative importance probably varies according to the prevailing environmental conditions.
The observation that chloroplastic MDA reductases and APXs are enhanced in antisense Arabidopsis (Arabidopsis thaliana) plants with suppressed chloroplast-located PRX suggests that regulatory compensation mechanisms exist between the pathways, so that one pathway may be enhanced to compensate for losses in the other pathway. Such observations of interactions between the AsA-GSH pathway and the PRXs demonstrate cross talk between the individual ROS-metabolizing pathways of the chloroplasts. The AsA-GSH pathway has a higher specificity for H2O2 and the chloroplast APX has higher activities than PRXs, but the PRXs have a broad specificity toward lipid peroxides and/or reactive nitrogen species, such as nitric oxide (NO). For example, values for the catalytic efficiencies (Kcat/Km; L mol−1 s−1) for plant 2-CysPrx with H2O2range between 2.5 × 104 and 1.8 × 103, with a value of 7.3 × 103 with tert-butylhydroperoxide. In comparison, the value for AsA-dependent H2O2 reduction is 0.9 × 106 L mol−1 s−1.
Like ROS, NO is an important plant signaling molecule. NO reacts rapidly with the superoxide anion to produce peroxynitrite. It is likely that peroxynitrite is produced in chloroplasts of turf grass leaves, where it may fulfill signaling functions. Moreover, in marked contrast to the situation in animals, peroxynitrite does not appear to be toxic to plants, which appear to function without problem even in the presence of high levels of this metabolite. A key feature of the regulation of NO metabolism is its reaction with GSH to form nitrosoglutathione, which can then react with other thiols to form nitrosothiols. Nitrosoglutathione serves as a storage pool of NO and probably also fulfills as yet unknown signaling functions. Like glutathionylation, protein S-nitrosylation can modify both protein function and activity.
While the AsA-GSH cycle operates largely within the stroma, it is also linked to the functions of a hydrophobic antioxidant, α-tocopherol (Toc), which is maintained in its reduced form by AsA. Carotenoids and tocopherols are the most abundant groups of lipid-soluble antioxidants in chloroplasts. Toc is accumulated to high concentrations in chloroplasts, where it serves to prevent lipid peroxidation by removal of singlet oxygen and lipid peroxyl radicals. The resultant tocopheroxyl radicals are reduced back to Toc by AsA and the action of the AsA-GSH cycle. The Arabidopsis vitamin E deficient1(vte1) mutants that are deficient in Toc show a significant accumulation of AsA and GSH. In contrast, VTE1-overexpressing plants, which accumulate Toc, have lower AsA and GSH levels. Such observations provide further evidence of the close relationships that exist between chloroplast antioxidants, which compensate for each other. Moreover, these data not only demonstrate the high degree of interaction between the chloroplast antioxidant pathways but also suggest that there are multiple sites of reciprocal control. However, while the biosynthetic pathways for AsA GSH and Toc are now well established, much remains to be understood regarding how the synthesis and accumulation of these essential antioxidants is controlled and regulated in a coordinated manner. The factors that control the intracellular partitioning of metabolites between the different compartments of the cell and the antioxidant transport systems are of particular importance to the overall regulation of photosynthesis and its effective operation over a wide range of environmental conditions. While recent years have witnessed an increase in our understanding of the roles of ROS in the signaling systems that coordinate antioxidant gene expression, very little information is available to date on how the low-Mr antioxidants participate in this control. Therefore, the balance between ROS production and ROS scavenging in chloroplasts is delicate and must be strictly controlled. AsA and GSH are the most abundant and best characterized water-soluble antioxidants in plants, and they accumulate to millimolar concentrations in chloroplasts. Although these metabolites scavenge ROS separately, they have long been considered to function together in the AsA-GSH cycle and the water-water cycle to metabolize H2O2 and to dissipate excess excitation energy in chloroplasts.
A common feature among the different ROS types is their capacity to cause oxidative damage to proteins, DNA, and lipids. These cytotoxic properties of ROS explain the evolution of complex arrays of non-enzymatic and enzymatic detoxification mechanisms in plants. Increasing evidence indicates that ROS also function as signaling molecules in plants involved in regulating development and pathogen defense response. Reactive oxygen species (ROS) are produced as a normal product of plant cellular metabolism. Various environmental stresses lead to excessive production of ROS causing progressive oxidative damage and ultimately cell death. Despite their destructive activity, they are well-described second messengers in a variety of cellular processes, including conferment of tolerance to various environmental stresses. Whether ROS would serve as signaling molecules or could cause oxidative damage to the tissues depends on the delicate equilibrium between ROS production, and their scavenging. Efficient scavenging of ROS produced during various environmental stresses requires the action of several non-enzymatic as well as enzymatic antioxidants present in the tissues. In this paper, we describe the generation, sites of production and role of ROS as messenger molecules as well as inducers of oxidative damage. Further, the anti-oxidative defense mechanisms operating in the cells for scavenging of ROS overproduced under various stressful conditions of the environment have been discussed in detail.
Production of Oxidative Stress in Cells
There are many potential sources of ROIs in plants. Some are reactions involved in normal metabolism, such as photosynthesis and respiration. These are in line with the traditional concept, considering ROIs as unavoidable byproducts of aerobic metabolism. Other sources of ROIs belong to pathways enhanced during abiotic stresses, such as glycolate oxidase in peroxisomes during photorespiration. However, in recent years, new sources of ROIs have been identified in plants, including NADPH oxidases, amine oxidases and cell-wall-bound peroxidases. These are tightly regulated and participate in the production of ROIs during processes such as programmed cell death (PCD) and pathogen defense.
An unavoidable consequence of aerobic metabolism is production of reactive oxygen species (ROS). ROS include free radicals such as superoxide anion (O2•−), hydroxyl radical (•OH), as well as non-radical molecules like hydrogen peroxide (H2O2), singlet oxygen (1O2), and so forth. Stepwise reduction of molecular oxygen (O2) by high-energy exposure or electron-transfer reactions leads to production of the highly reactive ROS. In plants, ROS are always formed by the inevitable leakage of electrons onto O2 from the electron transport activities of chloroplasts, mitochondria, and plasma membranes or as a byproduct of various metabolic pathways localized in different cellular compartments. Environmental stresses such as drought, salinity, chilling, metal toxicity, and UV-B radiation as well as pathogens attack lead to enhanced generation of ROS in plants due to disruption of cellular homeostasis. All ROS are extremely harmful to organisms at high concentrations. When the level of ROS exceeds the defense mechanisms, a cell is said to be in a state of “oxidative stress.” The enhanced production of ROS during environmental stresses can pose a threat to cells by causing peroxidation of lipids, oxidation of proteins, damage to nucleic acids, enzyme inhibition, activation of programmed cell death (PCD) pathway and ultimately leading to destruction.
Despite their destructive activity, ROS are well-described second messengers in a variety of cellular processes including tolerance to environmental stresses. Whether ROS will act as damaging or signaling molecule depends on the delicate equilibrium between ROS production and scavenging. Because of the multifunctional roles of ROS, it is necessary for the cells to control the level of ROS tightly to avoid any oxidative injury and not to eliminate them completely. Scavenging or detoxification of excess ROS is achieved by an efficient anti-oxidative system comprising of the nonenzymic as well as enzymic antioxidants. The enzymic antioxidants include superoxide dismutase (SOD), catalase (CAT), guaiacol peroxidase (GPX), enzymes of ascorbate-glutahione (AsA-GSH) cycle such as ascorbate peroxidase (APX), monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR), and glutathione reductase (GR). Ascorbate (AsA), glutathione (GSH), carotenoids, tocopherols, and phenolics serve as potent nonenzymic antioxidants within the cell. Various workers have reported increased activities of many enzymes of the antioxidant defense system in plants to combat oxidative stress induced by various environmental stresses. Maintenance of a high antioxidant capacity to scavenge the toxic ROS has been linked to increased tolerance of turf grass plants to these environmental stresses. Considerable progress has been made in improving stress-induced oxidative stress tolerance in crop plants by developing transgenic lines with altered levels of antioxidants. Simultaneous expression of multiple antioxidant enzymes has been shown to be more effective than single or double expression for developing transgenic plants with enhanced tolerance to multiple environmental stresses. The present review focuses on types of ROS, their site of production, and their role as messenger and inducer of oxidative stress. Further, role of antioxidative defense system in combating danger posed by overproduced ROS under stresses has been discussed in detail.
Reactive Oxygen Species, Sites of Production, and Their Effects
ROS are a group of free radicals, reactive molecules, and ions that are derived from O2. It has been estimated that about 1% of O2 consumed by plants is diverted to produce ROS in various subcellular loci such as chloroplasts, mitochondria, peroxisomes. ROS are well recognized for playing a dual role as both deleterious and beneficial species depending on their concentration in plants. At high concentration ROS cause damage to biomolecules, whereas at low/moderate concentration it acts as second messenger in intracellular signaling cascades that mediate several responses in plant cells.
Types of ROS
The most common ROS include 1O2, O2•−, H2O2, •OH. O2 itself is a totally harmless molecule as in its ground state it has two unpaired electrons with parallel spin which makes it paramagnetic and, hence, unlikely to participate in reactions with organic molecules unless it is activated. Activation of O2 may occur by two different mechanisms: (i) absorption of sufficient energy to reverse the spin on one of the unpaired electrons and (ii) stepwise monovalent reduction (Figure 1). In the former, 1O2 is formed, whereas in latter, O2 is sequentially reduced to O2•−, H2O2, and •OH.
Electrons in the biradical form of oxygen have parallel spin. Absorption of sufficient energy reverses the spin of one of its unpaired electrons leading to formation of singlet state in which the two electrons have opposite spin. This activation overcomes the spin restriction and 1O2 can consequently participate in reactions involving the simultaneous transfer of two electrons (divalent reduction). In the light, highly reactive 1O2 can be produced via triplet chlorophyll (Chl) formation in the antenna system and in the reaction centre of photosystem II. In the antenna, insufficient energy dissipation during photosynthesis can lead to formation of chlorophyll (Chl) triplet state, whereas in the reaction centre it is formed via charge recombination of the light-induced charge pair. The Chl triplet state can react with 3O2 to give up the very highly destructive ROS 1O2:Chllight−−−→3Chl,(1)3Chl+3O2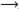 Chl+1O2,(2)
Chl+1O2,(2)
Further, limited CO2 availability due to closure of stomata during various environmental stresses such as salinity, drought favors the formation of 1O2. The life time of 1O2 within the cell is probably 3 μs or less. A fraction of 1O2 has been shown to be able to diffuse over considerable distances of




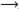 Chl+1O2,(2)
Chl+1O2,(2) 


