,
S
.9)
p
.
c
δ
Where δ is thickness of cement paste that film and glue aggregate’s grains; S
is total surface of aggregate’s grains.
Fineness and gradation of the aggregate make influence on formation of
structure and properties of no-fines concrete. Volume of voids between grains
also depends on cement content.
Unlike no-fines concrete, aerated lightweight concrete has porous structure
formed by component forming pores. By properties this type of lightweight
concrete takes intermediate place between concrete of dense structure and
cellular concrete. Forming pores of lightweight concrete mix permits to use
heavier porous aggregate without density increasing, to reduce quantity or to
refuse to use porous sand, to apply aggregate with gap grading. Raised
viscosity and workability are characteristic for aerated concrete mixes.
181





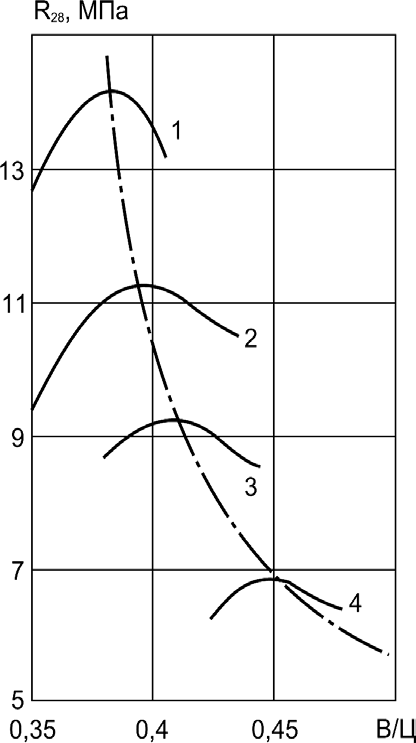
R28, MPa
Forming pores for concrete can be done by
foam, gas or air-entraining admixture.
Foam makes pores usually in noaggregates concrete, air-entraining
admixtures make pores in mixtures with
sand, gas – both mixtures with and without
sand.
0.35 0.4 0.45 W/C
Fig. 9.3. Relationship between
no-fines concrete strength at 28
day (R28) and water-cement
ratio (W /C):
1–concrete composition
(cement: gravel by volume) 1:6;
2–idem 1:7; 3–idem 1:8;
4–idem 1:10
182





9.5. Cellular concrete
Cellular concrete (gas concrete) has been suggested at first in 1889 by Czech
researcher Hoffman which used for mortars effervescence carbon dioxide. In
1914 Owlswort and Dyer (USA) were issued the patent on application of
aluminum and zinc powders to form hydrogen bubbles in cement stone, making
principles of modern gas concrete technology.
Cellular concrete is manufactured from binder, silica component, gas formers or
foaming agents and water. Both clinker and non-clinker (slag-alkaline and
others) cements, lime, gypsum are binders for cellular concrete production.
Cellular concrete is referred to mostly effective materials for enclosing
structures. At density 500-700 kg/m3 they permit to reach strength 3-5 МPа at
optimal structure. Basic factors of cellular concrete strength increasing at
keeping their density are more high fineness of components grinding and their
grading, thorough mixing, selection of optimal mixes compositions and curing
regime.
183





Aluminum powder is most common gas former. Powder adding provides start of
gas emission in alkaline environment after 1...2 min. Aluminum paste is used
along with powder. Gas forming reaction proceeds in following way:
Са
3
(ОН) + 2Al+ 6H O ⇔ CaO
3
⋅ Al O ⋅6Н О + Н
3
↑
2
2
2
3
2
2
As the result of chemical reaction from 1 g of aluminum at normal conditions
1.254 litres of hydrogen is formed, at 50°С hydrogen volume is 1.48 litres.
As foaming agents there are used different surface-active agents (sulphite
yeast, soap agent, etc.) and other substances, which at intensive mixing with
water make stable foams.
Cellular concrete strength (R ) correlates closely with its density (ρ ). Practice
c
c
for strength prediction of these materials there are used different empirical
equations, for example:
R = А 2
(9.10)



















