13.13
24.61
26.25
21
ISBN: 978-967-14475-3-6; eISBN: 978-967-14475-2-9 264
Biotech Sustainability (2017)
Biodiesel Production for Sustainability Meena Devi et al.
-ion hectare is classified as waste and
curcas is the most known variety; it
degraded land (Dwivedi et al., 2014).
requires little water or additional care;
therefore, it is adequate for warm regions
4.1. Typical oil crops useful for biodiesel
with little fertility. Productivity may be
production
reduced by irregular rainfall or strong
The main characteristics of typical
winds during the flowering season. Yield
oil crops that have been found useful for
depends on climate, soil, rainfall and
biodiesel production are summarized in
treatment during sowing and harvesting.
the following paragraphs.
Jatropha plants become productive after 3
or 4 years, and their lifespan is about 50
4.1.1. Castor seed
years. Oil yield depends on the method of
The castor oil plant grows in
extraction; it is 28–32% using presses and
tropical climates, with temperatures in the
up to 52% by solvent extraction. Since the
range 20–30◦C; it cannot endure frost. It
seeds are toxic, jatropha oil is nonedible.
is important to note that once the seeds
The toxicity is due to the presence of
start germinating, the temperature must
curcasin (a globulin) and jatrophic acid
not fall below 12 ◦C. The plant needs a
(as toxic as ricin).
warm and humid period in its vegetative
phase and a dry season for ripening and
4.1.4. Microalgae
harvesting. It requires plenty of sunlight
Microalgae have great potential for
and adapts well to several varieties of
biodiesel production, since the oil yield
soils. The total rainfall during the growth
(in liters per hectare) could be one to two
cycle must be in the range 700–1,400
orders of magnitude higher than that of
mm; although it is resistant to drought,
other raw materials. Oil content is usually
the castor oil plant needs at least 5 months
from 20 to 50%, although in some species
of rain during the year. Castor oil is a
it can be higher than 70%. However, it is
triglyceride, ricinolenic acid being the
important to note that not all microalgae
main constituent (about 90%). The oil is
are adequate for biodiesel production.
non-edible and toxic owing to the
High levels of CO2, water, light, nutrients
presence of 1–5% of ricin, a toxic protein
and mineral salts are necessary for the
that can be removed by cold pressing and
growth
of
microalgae.
Production
filtering. The presence of hydroxyl groups
processes take place in raceway ponds
in its molecules makes it unusually polar
and photobiological reactors.
as compared to other vegetable oils.
5. Biodiesel production techniques
4.1.2. Jojoba
Although jojoba can survive
There are different processes which
extreme drought, it requires irrigation to
can be applied to synthesize biodiesel
achieve an economically viable yield.
such as direct use and blending, micro
Jojoba needs a warm climate, but a cold
emulsion
process,
thermal
cracking
spell is necessary for the flowers to
process and the most conventional way is
mature. Rainfall must be very low during
transesterification process (Gashaw et al.,
the harvest season (summer). The plant
2015).
reaches its full productivity 10 years after
planting. The oil from jojoba is mainly
5.1.
Direct use and blending
used in the cosmetics industry; therefore,
The direct use of vegetable oils in
its market is quickly saturated.
diesel engine is not favorable and
problematic because it has many inherent
4.1.3. Jatropha
failings. Even though the vegetable oils
Jatropha is a shrub that adapts
have familiar properties as biodiesel fuel,
well to arid environments. Jatropha
it required some chemical modification
ISBN: 978-967-14475-3-6; eISBN: 978-967-14475-2-9 265
Biotech Sustainability (2017)
Biodiesel Production for Sustainability Meena Devi et al.
before can be used into the engine. It has
proportions. The equipment for thermal
only been researched extensively for the
cracking and pyrolysis is expensive for
past couple of decades, but has been
modest biodiesel production particularly
experimented with for almost hundred
in developing countries. Furthermore, the
years. Although some diesel engine can
removal of oxygen during the thermal
run pure vegetable oils, turbocharged
processing
also
removes
any
direct injection engine such as trucks are
environmental benefits of using an
prone to many problems.
oxygenated fuel. Another disadvantage of
pyrolysis is the need for separate
5.2.
Microemulsion process
distillation equipment for separation of
A micro emulsion is defined as the
the various fractions. Also the product
colloidal
equilibrium
dispersion
of
obtained is similar to gasoline containing
optically isotropic fluid microstructures
Sulphur which makes it less ecofriendly.
with dimensions generally in the range of
The pyrolyzed material can be vegetable
1–150 nm formed spontaneously from
oils, animal fats, natural fatty acids and
two normally immiscible liquids and one
methyl esters of fatty acids.
or more ionic or non-ionic. The problem
of the high viscosity of vegetable oils was
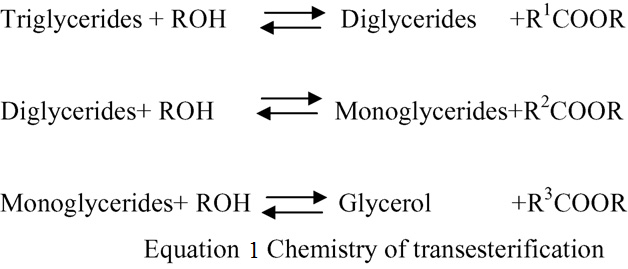
5.4. Transesterification
solved by micro-emulsions with solvents
Generally, biodiesel is produced
such as methanol, ethanol, and 1-butanol.
by
means
of
transesterification.
The components of a biodiesel micro-
Transesterification is the reaction of a
emulsion include diesel fuel, vegetable
lipid with an alcohol to form esters and a
oil, alcohol, surfactant and cetane
byproduct, glycerol. It is, in principle, the
improver
in
suitable
proportions.
action of one alcohol displacing another
Alcohols such as methanol and ethanol
from an ester, referred to as alcoholysis
are used as viscosity lowering additives,
(cleavage
by
an
alcohol).
In
higher alcohols are used as surfactants
Transesterification
mechanism,
the
and alkyl nitrates are used as cetane
carbonyl carbon of the starting ester
improvers. Microemulsions can improve
(RCOOR1) undergoes nucleophilic attack
spray
properties
by
explosive
by the incoming alkoxide (R O−) to give
2
vaporization
of
the
low
boiling
a tetrahedral intermediate, which either
constituents in the micelles. Micro-
reverts to the starting material, or
emulsion results in reduction in viscosity
proceeds to the transesterified product
increase in cetane number and good spray
(RCOOR2). Transesterification consists of
characters in the biodiesel. However,
a sequence of three consecutive reversible
continuous use of microemulsified diesel
reactions. The first step is the conversion
in engines causes problems like injector
of triglycerides to diglycerides, followed
needle sticking, carbon deposit formation
by the conversion of diglycerides to
and incomplete combustion.
monoglycerides,
and
finally
monoglycerides into glycerol, yielding
5.3. Thermal cracking (pyrolysis)
one ester molecule from each glyceride at
Pyrolysis is defined as the
each step. The reaction is represented in
conversion of one substance into another
equation 1.
by means of heat or heating with the aid
of a catalyst. Pyrolysis involves heating in
absence of air or oxygen and cleavage of
chemical bonds to yield small molecules.
The pyrolysis of vegetable oil to produce
biofuels has been studied and found to
produce alkanes, alkenes, alkadienes,
aromatics and carboxylic acids in various
ISBN: 978-967-14475-3-6; eISBN: 978-967-14475-2-9 266
Biotech Sustainability (2017)
Biodiesel Production for Sustainability Meena Devi et al.
There
are
different
acid catalyzed transesterification process,
transesterification processes that can be
which converts the FFA to esters (Leung
applied to synthesize biodiesel: (a) base-
and Guo, 2006).
catalyzed transesterification, (b) acid-
catalyzed transesterification, (c) enzyme-
5.4.3. Enzyme catalysts
catalyzed transesterification, and (d)
Lipase enzymes can also catalyze
supercritical alcohol transesterification.
methanolysis of triglycerides. The most
promising results were obtained by using
5.4.1. Catalysts: acid catalyst
immobilized Candida Antarctica lipase
The use of an acid catalyst is
(Novozym 435). Shimada et al., (1999),
observed to be more effective than alkali
found that Novozym435 was inactivated
catalysts when the concentration of free
by shaking it in a mixture containing
fatty acids is high. Also the performance
more than 1.5 M eq. of methanol to oil.
of the acid catalyst is not strongly
Above this concentration, methanol is
affected by the presence of FFAs in the
partially present as small droplets in the
feedstock. In fact, acid catalysts can
oil phase. These droplets are believed to
simultaneously
catalyze
both
cause enzyme deactivation. Therefore,
esterification
and
transesterification.
methanol was added stepwise; after the
Thus, a great advantage with acid
addition of the third methanol equivalent,
catalysts is that they can directly produce
conversion to methyl esters was almost
biodiesel from low cost lipid feedstocks,
complete. The enzyme could be reused 50
generally associated with high FFA
times without loss of activity. The
concentrations (low-cost feedstocks, such
occurrence of free fatty acids did not
as used cooking oil and greases,
affect the enzyme catalyst. Before the
commonly have FFAs levels of >6%)3.
inlet of every reactor,1 M eq. was added
However, Homogeneous acid catalyzed
to the feed. Samukawa et al. (2000)
reaction is about 4000 times slower than
reported a dramatic increase of the lipase
the homogeneous base-catalyzed reaction.
efficiency when it was pretreated by a
Acids used in the catalysis of the
consecutive incubation in methyl ester
transesterification
of
biodiesels
are
and oil prior to reaction. The use of
usually either hydrochloric acid or
Novozym435
in
methanolysis
of
sulfuric acid. Though these two acids are
triglycerides
is
also
reported
in
the most common, any Bronsted acid can
supercritical carbon dioxide at 24.1 MPa
also be used in this reaction.
and 50 ◦C. High yields (90–95%) of fatty
acid methyl esters could be obtained
5.4.2. Base catalyst
when the reaction was carried out at
Transesterification reaction can be
molar methanol/oil ratios of 25:1.
catalyzed by both homogeneous (alkalies
and acids) and heterogeneous catalysts.
5.4.4. Supercritical transesterification
The used alkali catalysts are NaOH,
Saka and Kusdiana (2001) have
CH3ONa, and KOH for producing
developed a catalyst free method for
biodiesel (Wang et al., 2007). The alkali
biodiesel fuel production by employing
catalyzed transesterification of vegetable
supercritical methanol. The supercritical
oils proceeds faster than the acid
treatment at 350 ◦C, 43 MPa, and 240 s
catalyzed. But the use of base catalyzed
with a molar ratio of 42:1 in methanol is
transesterification is only limited to oil
the
optimum
condition
for
having low water and FFA content. This
transesterification of rapeseed oil to
reaction is the most widely used process
biodiesel fuel. The great advantage of this
for production of biodiesel worldwide. To
method was that free fatty acids present in
keep check on the water and FFA content
the oil could be simultaneously esterified
of the oil, they are first pretreated with an
in the supercritical solvent. Variables such
ISBN: 978-967-14475-3-6; eISBN: 978-967-14475-2-9 267
Biotech Sustainability (2017)
Biodiesel Production for Sustainability Meena Devi et al.
as the molar ratio of alcohol to vegetable
found that reaction mixture containing
oil and reaction temperature were
65ml of methanol along with 2.4 g of
investigated during the transesterification
catalyst (KOH) took a good start in half
within this supercritical media. Increasing
an hour at30°C. In this reaction, amount
the reaction temperature within the
of glycerine removed as well as ester
supercritical regime resulted in increased
content
produced
was
considerably
ester conversion.
increased with rise in temperature of
mixture up to 70°C by extending time
6. Previous work done on production of
period (180-360 minutes). The removal of
biodiesel from edible oil
glycerine increased by two and half times
and ester content by four times,
Leung and Guo (2006) compared
respectively. When castor oil was
the transesterification reaction conditions
subjected to acid esterification, prior to
for fresh canola oil and used frying oil.
transesterification
(a
separate
Higher molar ratio (7:1, methanol/used
investigation), it was found that ester
frying oil), higher temperature (60° C)
contents up to 95% could be obtained.
and higher amount of catalyst (1.1 wt%
Hasan et al. (2013) produced biodiesel
NaOH) was maintained in used frying oil
from neem seeds, its properties were
when compared to fresh canola oil where
close to diesel. The methodology of
optimal conditions maintained were 315-
esterification process was selected and
318 K, 1.0 wt% NaOH and 6:1
carried out by 1000 ml raw neem oil,
methanol/oil molar ratio. However, less
300ml methanol and sodium hydroxide
reaction time (20 min) was observed for
on mass basis as a catalyst usually kept in
used frying oil when compared to fresh
oven to form methyl ester, and initially to
canola oil reaction time (60 min). Ying et
reach
equilibrium
condition
at
al. (2011), developed a new method
temperature 55-66°C. The ester and
catalyst,
benzyl
bromide-modified
glycerine were separated by stimulating
calcium oxide (CaO) for production of
continuously and allow settling under
biodiesel from rapeseed. The improved
gravity for 24 h. Thus the separated ester
catalytic activity was obtained by better
contains 3% to 6% methanol and soap
fat diffusion to the surface of the benzyl
agents. The methanol was removed by
bromide-modified CaO. Further, a 99.2%
vaporization. The biodiesel had some
yield of fatty acid methyl esters in 3h was
catalyst; it was removed by warm water
obtained in comparison to by better fat
mix with ester. Kinematic viscosity lay
diffusion to the surface of the benzyl
between 1.9 and 6.0 according to the
bromide-modified CaO. Wakil et al.
ASTM D6751 specification. It was
(2012), chosen Cottonseed oil, Mosna oil
reported that, 0.95 L biodiesel was
and Sesame oil for producing biodiesel.
produced from 1 L neem oil.
7. Previous work done on production of
8. Factors affecting biodiesel
biodiesel from non-edible oil
production
Mohibbe et al. ( 2005), found that
The yield of biodiesel in the
FAME of Jatropha curcas were most
process of transesterification is affected
suitable for use as bio- diesel and met the
by several process parameters which
major
specification
of
bio-diesel
include;
reaction
time,
reaction
standards of the European, Germany and
temperature, catalyst and molar ratio of
USA
Standards
Organization.
alcohol and oil and mixing intensity
Chakrabarti and Ahmad (2008) presented
(Gashaw et al., 2015).
work on extraction of oil from castor bean
and converting it into biodiesel. It was
8.1. Temperature
ISBN: 978-967-14475-3-6; eISBN: 978-967-14475-2-9 268
Biotech Sustainability (2017)
Biodiesel Production for Sustainability Meena Devi et al.
Reaction
temperature
is
the
of alcohol and 1 mole of triglyceride are
important factor that will affect the yield
required for transesterification to yield 3
of biodiesel. For example, higher reaction
moles of fatty acid methyl/ethyl esters
temperature increases the reaction rate
and 1 mole of glycerol is used. In order to
and shortened the reaction time due to the
shift the reaction to the right, it is
reduction in viscosity of oils. However,
necessary to either use excess alcohol or
<




