Chapter 3
Understanding Heat
Heat is a form of energy. When you bask under the sun, you feel its heat, you get warm. Sun is providing you some energy which your body uses to maintain a temperature good enough to keep you warm. What is temperature? Take any two bodies, one sees that one of the bodies is relatively cooler than the other. Consider earth and sun. Sun is hotter than our earth. Take a more familiar example: Take a hot cup of tea and touch it with your hands. Now before touching, your hand was cooler. But slowly, as you keep touching the hot cup, your hand starts to get hotter than it was before.

Sometimes, we are in hurry. I am prone to this behavior too. So, sometimes, when the food is still hot, I (without knowing the temperature of the chapattis) take them in. My mouth burns and I look for water. But then, I realise that slowly as I keep eating hot food, temperature of my mouth and that of the food will equal. I mean to say that when I had not taken any hot food inside my mouth, it was at relatively lower temperature than the food. Thus, when hot food was taken in, my mouth could not bear the new increase in temperature. It takes time for the difference to decrease.
So I have defined temperature, but indirectly. Temperature is the measure of how hot or how cold a body is. It is not heat. When you say: “This body has high temperature” – you are wrong. This is because temperature is relative. A cup of tea is hot only relative to your hand. It is not hot relative to sun. I hope you understand the difference. So what is heat?
I say it again. Heat is a form of energy. But why does heat exist at all? Where does it come from? Why is one body hotter than the other? A good answer comes to my mind. When there is a difference in temperature between two bodies, heat transfer occurs between them. Since the body that is hotter of the two has excess of heat, it will want to decrease its energy and become stable. The other body will gain some heat and become hotter than it was before. The transfer occurs until there is equilibrium.
What is equilibrium? It is a state of equality. You keep a hot cup of tea on your study table. But some way or other, you forget to consume it. The cup does not care for you. It will lose heat. The surrounding just close to the cup is cooler relatively. Nature does not like this, nature wants harmony. Eventually, your hot cup of coffee will get cooler. It cannot store heat forever!
What are we trying to talk about? So far, we know that heat is a form of energy. Temperature is a degree of measure of hotness or coldness and that heat transfers from a hot body to a cold body. We are yet to talk about equilibrium.
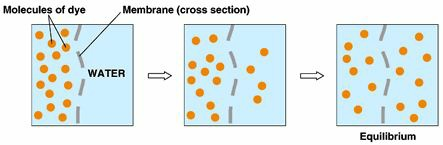
Take a tank. Divide this tank into two parts. Fill one of the halves completely with water. The other half should remain empty. Now remove the partition between the two halves. What will happen? You say that it is obvious that the water from the one half will distribute equally between the two. This is obvious, isn’t it? With heat, this is obvious too.
Now think about particles. Think about gases. Take a tank and it has air inside. Air has lots of small particles that we cannot see. These particles or atoms move around randomly. Why do they move? They have thermal energy. But if we heat this tank, we provide the energy to the tank. The atoms would speed up. Their thermal energy will increase.
What am I trying to say? You may ask. I am trying to show you how the world works. You heat an ice cube. It will turn into water, eventually. You heat water, and it turns into steam, only eventually. Why do these conversions happen? Because of heat that you give. In a solid, atoms are close together, they are tightly packed. Thus the random movement is not there. There is only small vibration of atoms about their positions. When you heat a solid, these vibrations increase. As you continue heating the solid, these vibrations grow so stronger that they overcome the force that was supposed to hold the solid. The solid breaks and turns into liquid. In liquids, there is some movement. Unlike solids, liquids can flow. When you heat a liquid, you provide energy to its constituents and eventually, they become free. Liquid turns into gas. Gas is that state of matter in which constituents are no longer bounded, they are ultimately free from all bounds and move around randomly. Heat is responsible for the changes that you see in your immediate environment. Consider evaporation. You may have studies about evaporation when you were younger. Do you still remember it? It involves heating. In fact, evaporation speeds up upon heating. The surface molecules get heated first, thus they are the ones which turn into gases. Evaporation is a surface phenomenon. It is a continuous process. The question that comes ahead is: “If rivers and oceans are always being evaporated, will they get empty some day?” The answer is not obvious as you may think.
You think you know it. You say that evaporation and raining are two simultaneous processes. You say that if sea water gets evaporated, it will return as rain someday. You seem to be correct. Nature loves harmony. But have you heard of stories that rivers dry too? Suppose we have river ‘A’ in city ‘C’. But this city is a strange city. Evaporation occurs but it is one of the driest regions in the country (this is obviously possible too!) The river evaporates completely in 100 years (just to give you an idea) and it rains only once in 10 years (a very dry city) – what do you think will happen? Do you see what I am trying to point out? The river will someday vanish. Did the same thing occur on the planet Mars? Try to think about it!
What are the uses of heat? What keeps you warm? Heat! Mars is too cold to sustain life. Mercury is too hot. One needs a perfect location. It just so happens that our planet earth is set in the perfect spot. Heat energy is very necessary and I do not need to tell you the examples. In the next chapter, we will learn about energy. What is energy? Let us understand...





