Chapter Seven
7. THE COMMANDMENTS OF STUDYING INTEGRAL MEMBRANE PROTEINS
Raymond J. Turner *
Department of Biological Sciences, University of Calgary
Calgary, AB, Canada
ABSTRACT
As a budding biochemist, I was introduced to Arthur Kornberg's ten commandments of enzymology. After 25 years of working in the field of integral membrane protein (IMP) structure-function, my trainees and students of my class noticed that I would make statements on IMP research in the form of a commandment, in the guises of these early biochemistry commandments. Here I share my commandments around IMP expression and purification, IMP biochemistry, IMP functionality studies, and IMP high-resolution structures.
Keywords: integral membrane proteins, expression and purification, detergent solubilization, membrane mimetics, structure and function, biochemistry, hydropathy analysis, membrane topology, membrane sidedness
7.1. INTRODUCTION
A favorite read I give all my students on the introduction to the topic integral membrane protein (IMP) biochemistry is the story of a novice membrane protein biochemist and how he learns to love lysozyme (von Heijne, 1999). This article is an informative yet playfully written review by Gunner von Hejine, where he good-humouredly highlighted the frustrations encapsulated in being a biochemist trying to study integral membrane proteins. Although considerable advancements in technology have occurred in the past 10 years since this review was written, IMPs are still notoriously more difficult to study than their soluble cousins.
This chapter aims to highlight some of the tripping points and mistakes to avoid or at least to be aware of, for those embarking down a path of study on an IMP. To deliver these thoughts in a highly consumable fashion, I will try to channel the great Arthur Kornberg’s Ten Commandments of Enzymology (Kornberg, 2000, Kornberg, 2003), which I also consider mandatory reading for every budding biochemist. Each commandment is stated and then explained in the context of understanding IMP structure-function.
_________
* Direct all correspondence to Prof. Raymond J. Turner; Department of Biological Sciences, 447 Biological Sciences Building, University of Calgary, 2500 University Dr. NW, Calgary, AB, Canada, T2N 1N4. E-mail: turnerr@ucalgary.ca.
7.2. THE COMMANDMENTS
THOU SHALT…
7.2.1. COMMANDMENTS AROUND IMP EXPRESSION AND PURIFICATION
7.2.1.1. Not strive to OVER-express integral membrane proteins (sic)
Well let’s just say it, over-expressing IMPs can be frustrating at best. But let’s correct this wording; what is really meant here is enhancing functional protein accumulation and in this chapter we will refer to it as EFPA. Unfortunately, maximizing protein accumulation has been foolishly and incorrectly called “protein over-expression” for decades, leading to disgruntled comments from molecular biologists gleefully pointing out how dimwitted protein biochemists can be in their fundamental understanding of biology. This of course is not limited to IMPs, but all EFPA. So, let’s not continue to propagate this language. Proteins do not express, genes are expressed, subsequently producing mRNA, which is further translated into proteins. Thus, for EFPA to occur, we need several steps to be working at their optimal levels and properly coordinated. The process is far more complex than we typically consider, as not only does a protein have to be efficiently translated but in many cases, they are assisted in their folding, cofactors need to be added, they require cellular targeting as well as potentially assisted to assemble into a multiprotein complex. In the case of IMPs, ‘properly targeted’ means to the membrane (and the correct membrane in the cell), inserted into the membrane in the correct topology and post insertional folding and transmembrane segment assembly/interactions to occur (for a more complete description of these processes see the review by Cymer, von Heijne and White [2015]).
EFPA is notoriously challenging with IMPs as the volume of protein capture in a cell (the membrane itself) is considerably more limiting than the cytoplasm, particularly when we consider prokaryotic expression systems. EFPA of IMPs is typically plagued with problems of protein aggregation and inclusion bodies through the transmembrane helices (TMH) miss folding into beta-strands. Additionally, misfolded or slowly folding proteins will have exposed regions leading to degrees of proteolytic degradation (Schlegel et al., 2014).
Expression and translation at a high rate for some proteins can lead to high cellular stress. EFPA of a transporter can lead to uncoupling of the proton motive force (Winstone, Duncalf and Turner, 2002) or miss directed and non-specific transport across the membrane, both of which can lead to cellular stress or lethality. The stress of producing the IMP can actually lead to loss of expression through the selection of cells that are producing less IMP and thus more physiologically fit. This fitness selection can lead to plasmid curing and expression loss within yeast and bacterial expression systems.
Here it is also important to have some words about heterologous IMP expression. We observe that often IMPs do not have their codons optimized. The use of rare codons may be present to decrease the translation rate to coordinate more closely with the folding, targeting and insertion of the protein. Thus, methods to optimize codon usage for the expression of IMPs can lead to folding and assembly problems and reduce IMP production (Schlegel et al., 2012). However, recently groups have gone after this issue exploring optimization of transcription rates and codon usage, which appears promising (see Claassens et al. (2017) and citations within for work in this area).
Overall, considerable time, effort, resources, and ‘luck’ must be put forward to optimize EFPA of IMPs as there are no effective rules of thumb. Of course, if going after a homolog or similar IMP family member, where previous work has provided a template toward EFPA, it is worth starting with such information and optimizing around it. But, for the most part, EFPA of IMPs is imperially determined. Thus, it should be considered (but is more often overlooked), that it is often more resource-efficient to accept the low initial yields one may start to get and simply do multiple preparations to get enough material for targeted experiments. Yet optimizing EFPA can be a wonderful learning experience for a graduate trainee, and gleefully fun for those that enjoy dancing with experimental conditions.
7.2.1.2. Not to forget about the detergent
Throughout this chapter, I use the term 'membrane mimetic' rather than just detergent, as recently, alternative compounds have been explored to overcome issues with classical detergents. Regardless, most IMP researchers still use detergents (surfactant, amphiphiles, tensides, soaps) to solubilize their IMP of interest from their lipid home. To date, finding the best membrane mimetic is still mostly empirically determined, and various detergent companies now provide small samples in detergent array kits to explore detergent ‘space’ for your IMP of interest. Yet there are some things to consider. A key thing to remember is that one is trying to mimic the natural lipid environment as closely as possible.
Detergents are made up of essentially two parts. 1) The head, which is polar and can be anionic, cationic, zwitterionic, or non-ionic. 2) The tail, which is hydrophobic and can be flexible, straight, branched, or more ridged and steroidlike. Important considerations of working with detergents are overviewed in Tables 1 and 2.
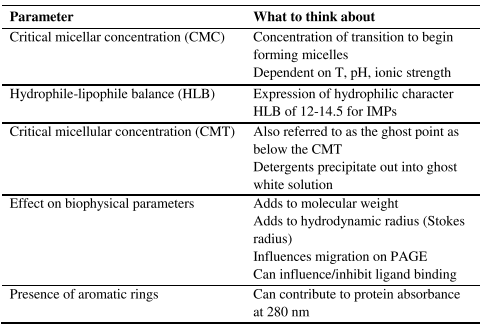
Table 1. Important parameters of detergents.
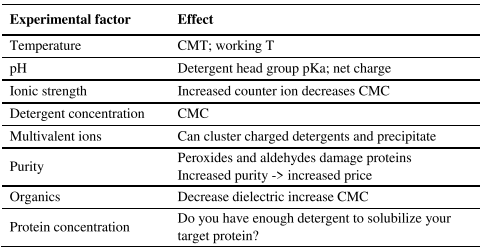
Table 2. Factors that affect detergent performance.
Alternatives to the use of classical detergents are still limited. Amphipols have been explored for some time, which are essentially polar or charged polymers decorated with acyl chains which are thought to wrap around the nonpolar domains of IMP providing aqueous solubility (Popot, 2010). Attempts to use perfluorinated surfactants have shown some success as they can maintain IMP lipid contacts (Popot, 2010). Another interesting group is the maltose neopentyl glycol amphiphiles, which gives two heads and two tails linked (Chae et al., 2010). The detergent alternative that has received the most hype recently are SMALPs. These are a Styrene Maleic Acid copolymer (SMA), which is considered to extract the IMP along with a small amount of the natural lipid bilayer encircled by the polymer to generate SMA Lipid Particles. SMALP scaffolded IMPs have quickly become popular with structural biochemists, particularly those using electron microscopy (Knowles et al., 2009; Postis et al., 2015).
Issues around the choice of detergent that are often overlooked include that the IMP may have considerably different thermal and kinetic stability when solubilized in detergent compared to its natural lipid bilayer. Further, the detergent may not be a ‘neutral’ player and could potentially act as an inhibitor or allosteric regulator to IMP activity.
To avoid issues around biochemical experiments in detergents, IMPs can be reconstituted into lipid bilayer (typically of defined lipid content) vesicles to try to return to a natural state. Here one must consider the size of the vesicle and if membrane curvature could influence the biochemistry of the IMP of interest.
A few tricks exist to avoid the use of amphiphiles for purifying the IMP with its natural lipids. Along with SMALPs, bicelles (Sanders and Prosser, 1998) and lipid nanodisks (Baybur and Sligar, 2010) allow for natural lipid bilayer encapsulation but they are small enough for structural studies. An approach to avoid detergent and lipid completely has been introduced by expressing the IMP as a protein-peptide fusion construct referred to as the SIMPLEx system (Mizrachi et al., 2015). The IMP remains soluble and folded correctly with the amphipathic peptide providing a hydrophobic shield around the IMP.
Regardless of which membrane mimetic is chosen one needs to remember that it is NOT the natural environment of the IMP and changes to dynamics and stability will occur. Even with the lipid-based systems of SMALPs, bicelles, and nanodisks, the lateral pressure and diffusability will be different, and of course, the sidedness of the protein will be lost. Additionally, one should consider that detergents can have deleterious interactions with the extramembranous soluble domains (Yang et al., 2014). It is fundamental to remember that a solubilized IMP is a fish out of water, still a fish, but its behaviour can be very different.
7.2.1.3. Not to overlook the additional challenges of IMP purification
For IMPs the first challenge is optimizing EFPA, but one typically wants to purify the protein for structure-functional studies. For the most part, one can follow the plethora of protein purification approaches used for soluble proteins (see Deutscher [1990] as a classical excellent resource). As with soluble proteins, one must be concerned about the variables of cell lysis approaches and the protection of protein from proteases and oxidative stressors. Yet, the choice of temperature, buffers, and metal/salt ions and concentrations becomes more complicated due to the need to use membrane mimetics to solubilize the protein (see other commandments as well).
The factors highlighted in Tables 1 and 2 can lead to serious issues in protein purification as the net charge of the protein is influenced by the charges of the detergent and the size of the protein is increased with the detergent shell. Thus, chromatographic purification approaches behave considerably different as well as how the protein will behave when centrifuged. A common error in purifying a detergent-solubilized IMP with ion-exchange chromatography is forgetting the net charge of the head group of the detergent and only focusing on the calculated pI of the target protein. The reader is directed to sections I and II of Hunte, von Jagow and Schagger (2003), for examples and more detail on the considerations of each purification method.
An extremely popular approach to facilitate protein purification is to add an affinity tag through molecular biology approaches. The most frequently used tag is that of the hexa-histidine (His6) peptide added for use on immobilized metal ion absorption/affinity chromatography (IMAC). Undoubtedly, affinity-affinity tags (Terpe, 2003) have been the most significant technological advancement helping accelerate protein biochemistry. However, there are issues with such tags influencing and changing the activity and stability of the tagged target protein (Majorek et al., 2014; Mohanty and Weiner, 2014; Booth et al., 2018). An example of the influence of a His6 tag on an IMP transporter is seen with the bacterial multidrug resistance transporter EmrE where the in vivo resistance profile was changed as well as changes of in vitro structural observations regarding multimeric state equilibrium and ligand binding upon the addition of the tag (Qazi et al., 2015). Therefore, one can still utilize affinity tag approaches to initiate the purification, but do not consider that your protein will behave as wildtype in vivo or reconstituted and that the tag may lead to miss-interpretations in structural and functional studies.
7.2.2. COMMANDMENTS AROUND IMP BIOCHEMISTRY
7.2.2.1. Understand the differences in stability compared to globular proteins
IMPs are remarkably thermodynamically stable in the lipid bilayer. This comes from the hydrogen bonds fully satisfied in the hydrophobic environment where the weakly coulombic nature of the H-bond is enhanced in the hydrophobic environment. Similarly, salt bridges are stronger and the intimate contacts of the van der Waals interactions between helices provide large enthalpic contributions in addition to the balance of the lipophobic effect and hydrophobic matching towards entropy contributions (White and Wimley, 1999; Engelman et al., 2003; Bowie, 2005).
The mixed solvation conditions (lipid environment and bulk aqueous phase) leads to unexpected behavior in traditional biochemical protein manipulation conditions. Consider denaturation using urea or guanidinium ion. Both of these denature a protein by competing out H-bonds. In the case of an IMP in a lipid or membrane mimetic, the extra membrane domains will denature but the transmembrane polypeptide remains inaccessible to these compounds and does not denature. Thermal denaturation is different as well due to the large enthalpy and many IMPs do not thermally denature. If they do, upon cooling they often aggregate into beta-strand based amyloid structures. Certainly, the delipidated protein is highly unstable and the reason why IMPs are manipulated in membrane mimetics.
A traditional storage approach of proteins is lyophilization, or -20°C storage. Lyophilisation can concentrate salt ions leading to aggregation events with detergents. The freezing step can trap crystallization water within the detergent protein complex dissociating TMHs leading to protein aggregation. As with any protein storage conditions, tests for protein aggregation need to be conducted.
Many small IMPs with short extra membrane loops do not denature (linearize) in sodium dodecyl sulfate (SDS). In fact they can maintain their structure and ligand binding properties in such detergents (Tulumello and Deber, 2012). The use of this detergent in electrophoresis techniques is standard biochemistry and migration comparisons are based on the ratio of SDS molecules with molecular weight to define a charge to mass ratio. For IMPs, they can bind differential amounts of SDS (depending on the degree of TMH-TMH interactions are disrupted) leading to anomalous migration on SDS-PAGE (Rath et al., 2009). There is also the issue of the competitive ability of SDS to outcompete the membrane mimetic used for the solubilization steps.
Tips to deal with these issues experimentally are provided in Hunte, von Jagow and Schagger (2003), but overall it is important to recognize stability differences compared to globular proteins.
7.2.2.2. Think carefully of experimental conditions
Biochemists will think carefully of their experimental physicochemical conditions: pH and buffering compound, ionic strength, counterions, dielectric constant, redox potential, temperature, protein concentration/density. These conditions are chosen with their protein of interest in mind to try to mimic the natural physiological conditions as well as stabilize their system while doing experiments. In this regard, we work in a biological buffer with a mixture of various compounds and concentrations to define the ideal condition.
However, when working with IMPs we have an additional compound, the membrane mimetic, which is either influenced or influences the other biological buffer components. If we don’t want our detergent, and subsequently our IMP, precipitating out we need to consider how the biological buffer components will influence the detergent performance (consider Tables 1 and 2).
Temperature is likely the most frequent mistake of the novice IMP biochemist in the lab. This comes from the standard of doing all protein experiments on ice or in the cold room. Yet, many detergents have their CMT between 4 and 24 °C, which leads to the detergents crystalizing and precipitating out or forming gel-like phases.
Another consideration is in moving from the detergent or detergent-like compounds being used as membrane mimetics to reconstitution into proteoliposomes. Considering first that reconstitution is an art form in itself and this process is often different for each unique IMP. Then one must consider the lipid composition of the liposome as well as the size. Will curvature strain be an issue for ones given IMP; i.e. does it originate from a membrane with high curvature or more planar? Or more fundamentally, are your chosen lipids appropriately hydrophobically matched (Killian, 1998)? Even considering the head group and lipid mixture is of importance regarding maintaining topology. Biological lipids have different lipid compositions in each leaflet of the bilayer and protein sequences have evolved to match.
It is extremely difficult, if not impossible to mimic accurately all the biological conditions. So beyond knowing this as a biochemist, here I leave the warning to be extremely careful about the interpretation from your model system as to what is going on in the cell.
7.2.3. COMMANDMENTS AROUND IMP FUNCTIONALITY STUDIES
7.2.3.1. Not to ignore ligand binding differences
Kinetics and diffusion in three-dimensional space are considerably different than in the two-dimensional plane. Here we consider the differences in the ability to bind ligand. The ability of a substrate to find the binding site of an IMP transporter or receptor in a lipid bilayer will be different than when it is solubilized in a membrane mimetic allowing extra degrees of freedom. This will affect the ‘on rates’ and subsequently the binding constant. Differences in this diffusion property on the kinetics are complemented by differences in thermodynamic energy properties of ligand-protein binding. The dynamics of the peptide chain and subsequent movements of amino acid side chains would be modulated differently by the membrane mimetic compared to the lipid bilayer.
Thus the on/off rates can be different leading to very different binding constants. An early study evaluating nucleoside transporters noted that different detergents needed to be screened to find one that got close to retaining the high ligand affinity observed in vivo (Hammond and Zarenda, 1996). A study illustrating these differences of membrane mimetic on structure and dynamics, circular dichroism and fluorescence were performed on the model multi-ligand transporter, EmrE. Remarkable differences were observed with the protein solubilized in different detergents as well as vesicle systems and solvents (Federkeil et al., 2003). A follow-up study using isothermal titration calorimetry confirmed the above statements with Kd values for the same substrate ranging over 10 fold (Sikora and Turner, 2005). A more recent example is seen with Pglycoprotein, where modulators stimulated ATPase activity compared to inhibition in native membranes (Shukla et al., 2017).
The issues around detergent selection for maintaining activity have been recognized since the 1960s with studies of different detergents on mitochondrial enzymes (for example see Soltysiak and Kaniuga [1970]). Unfortunately, such issues became forgotten with high throughput purification methods, as there are very few studies evaluating changes to the structure, binding with a change of membrane mimetics. Yet this issue is found in various early texts on membrane proteins (early attention to this issue brought up in Gennis [1989]). Few would do activity comparisons under different solubilization conditions as, well, why would they? If after a frustrating timeframe optimizing EFPA and detergent selection and purification, finally finding something that worked, few would want to start again.
So this commandment is simply to note that there is a strong likelihood that different solubilization and physicochemical conditions will give different answers about your IMP of interest.
7.2.3.2. Remember that membrane sidedness is lost
This seems obvious, but it tends to be forgotten or ignored. Upon solubilization of an IMP in a membrane mimetic, it is important to recognize that the biological sidedness of the protein has now been lost. The biological membrane is referred to in most textbooks as a semi-permeable barrier and divides different environments. Consider the protein in Figure 1. Each side of the membrane has completely different physicochemical environments. There can be remarkable differences in pH, ionic strength, specific ions and ion concentrations, dielectric constant, redox potential, and both low and high molecular weight metabolites and biomolecules (peptides, proteins, carbohydrates, nucleic acids). There will also be differences in fluidity in the lipid leaflet on each side of the membrane with conditions of side influencing the protein dynamics, ligand binding and catalytic activity.
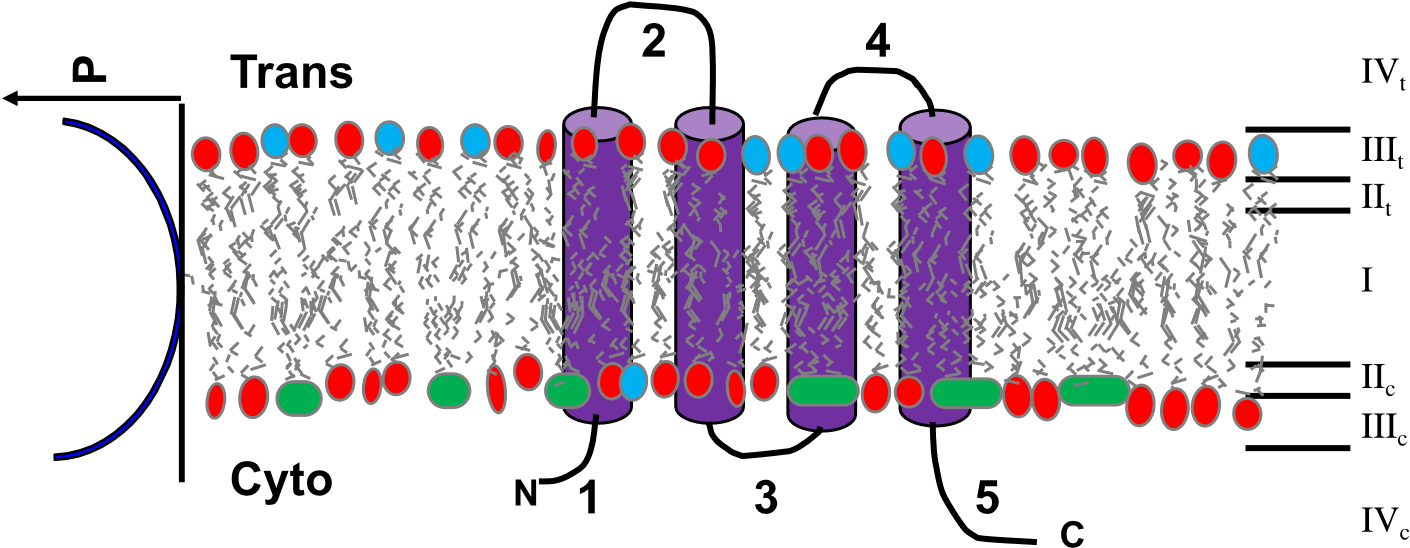
Figure 1. Depiction of a biomembrane with a protein topology sketch. Cartooned here is a bilayer demonstrating a different lipid composition between the two leaflets of the membrane. The two sides of the membrane are defined as the cytoplasmic side or cis side (subscript ‘c’) and the outside or trans side (subscript ‘t’). On the right in roman numerals indicates the different regions of the membrane: I as the acyl chain lipid core; II as the slightly polar glycerol region of the glycerol lipids; III as the highly polar and/or charged lipid head group region potentially also influenced by a phosphoester bridging group; IV the bulk solvent. The subscripts of these regions are to depict that their physicochemical environments are different to each other. Considering the potential of differential saturation of acyl chains, once could also divide region I to the c and t leaflets as the dynamics and packing pressure can also be different. The graph depiction on the left illustrates the nature of the hydrophobic (polarity; P) gradient that exists, and that it is not an instant transition. A topology model of a 4 transmembrane helix protein is also shown with the numbers indicating the different extra membrane loops. From this diagram one should recognize that the chemical environments of loops 1, 3, and 5 would be different from loops 2 and 4.
Biologically, an IMP has evolved so that the amino acid sequence on the different sides of the membrane is well suited to those specific physicochemical conditions. Thus, once the protein is solubilized, the extramembrane domains are now exposed to identical conditions, defined by the experimentalist, which may or may not be relevant to either side of the membrane. Replacing detergent membrane mimetics with bilayer disks can solve the issues around the accuracy of the detergent to replicate the bilayer, yet the sidedness is still lost leaving the extra membrane domains exposed to the same conditions.
7.2.3.3. Not to confuse in vitro vs in vivo activity
I have been amused over the years receiving reviewer comments asking for activity measurements of the detergent-solubilized transporters I have worked on. Given that a transporter moves a ligand from one side of the membrane to the other, such activity by definition is lost once solubilized. A similar issue exists for ion channels and receptors. This is the ramification of the biomembrane sidedness and solubilization causing forfeiture of physicochemical condition separation as in the comments above. One can still measure ligand binding as a proxy, but the binding observed will likely be somewhat disconnected to the in vivo state. Not to say such experiments are not worth doing to compare mutants, ligand specificity, and inhibitors.
For the most part, it is still impossible to obtain biochemical structurefunction information of an IMP while in its natural cellular environment in vivo. Certainly, experiments in proteoliposomes, defined detergent vesicle, or black membrane systems provide closer to in vivo realities, but may still not be able to provide exactly replicated conditions. Yet combining genetic phenotype experiments beside good cell biology, complemented with in vitro biochemical and structural studies a considerable wealth of knowledge has been amassed on IMPs. The message here is simply to recognize that our experimental model systems still do not precisely mimic those of a cell.
7.2.4. COMMANDMENTS AROUND IMP STRUCTURE
7.2.4.1. Not to put all faith in hydropathy plots
I still find myself shocked when in a graduate student committee meeting where they are announcing they have cloned a gene responsible for this or that, and they had performed bioinformatic analysis and go on to define it as an IMP and show me a picture of the TMH winding back and forth through a double-lined membrane followed by excitement around some domain of residues as binding site or the like. Such meetings remind me of the blind faith students (and senior researchers) often have in some of our tools, using them as black boxes. But in the case of IMPs, not understanding the issues around hydropathy analysis and various prediction algorithms frequently leads to wrong models and subsequent downstream problems in experimental interpretations. It is not to say many of the present programs work remarkably well, however they may never be 100% accurate.
The first comment on this issue is the choice of hydropathy scale that is applied to the primary amino acid sequence. For the uninitiated, this first step is considered trivial. Assign a value of hydrophobicity to each amino acid and apply these values to the primary sequence as initially applied by Kyte and Doolittle (1982). However, it is remarkably challenging to agree on such a value for each amino acid. More than 80 hydrophobicity/hydrophilicity indices existed by 1989, and the next two decades saw on the order of 2-4 new hydropathy scales proposed per year. Some became favored for various regions, but often would be chosen by default or random and applied to a sequence to generate hydropathy plots predicting TMH regions, which were taken on faith to be accurate, but overall were disappointingly error-prone (Elofsson and von Heijne, 2007).
Advancements came as the field recognized that some classes of IMP were more efficiently






