Extension
Ask students to find current event stories in newspapers, magazines, or Activity
television programs that talk about chemical exposure. Challenge students to find one event that involves a chemical exposure that harms humans
or other living things and one that involves a chemical exposure that
benefits humans or other living things.
You will be able to use a chemical exposure described in these articles in the extension activity in Lesson 5.
Tip from the field test: If students in your school are required to bring in current event articles for several other classes, coordinate with teachers making similar assignments so that students are not duplicating efforts.
Alternatively, collect articles yourself and display them in the classroom.
40
L E S S O N 2
Explore
The Dose
Makes the Poison
Overview
At a Glance
Students observe beakers of water that contain different amounts of a
mystery chemical. They discuss how each amount of the chemical might
affect them if the chemical was beneficial or harmful to their bodies.
Then, students set up investigations to test the effects of different doses of chemicals on seed germination and collect data for two consecutive days.
Their investigations model the kinds of investigations toxicologists do to determine dose-response relationships in living systems.
Major Concepts
The total amount of chemical administered to, or taken by, an organism is called a dose, and the effect a chemical has on a living organism is called the response. The effect a chemical has on a living organism is related to its dose and the resultant concentration of chemical in the organism. Toxicity tests enable toxicologists to learn about responses of living organisms to doses of chemicals.
Objectives
After completing this lesson, students will
• recognize that the total amount of a chemical administered to, or taken by, the organism is called a dose,
• understand that the effect a chemical has on a living organism is called the response,
• recognize that the effect a chemical has on a living organism is related to its dose and the resultant concentration of chemical in the organism, and
• demonstrate how toxicity tests enable toxicologists to learn about
responses of living organisms to doses of chemicals.
41
Chemicals, the Environment, and You
Background
Dose, Concentration, and Threshold
The beneficial and harmful effects that a chemical has on an organism
Information
depend, in part, on the amount of the chemical that gets into the organism.
The total amount of a chemical that is administered to, or taken by, the
organism is called the dose. The effect of a chemical depends not only on the amount of the chemical that gets into the organism but also on
the resulting concentration of the chemical in the body (the amount of chemical compared with the body size), the length of exposure to the
chemical, and the route of exposure.
The measure of dose in toxicology is important; a large dose of a beneficial chemical can have a harmful effect, and a small dose of a harmful chemical can have no adverse effect. In the words of the 16th-century physician
Paracelsus, “All substances are poisons; there is none which is not a poison.
The right dose differentiates a poison from a remedy” (Klassen, 2008).
Approximate Lethal Doses of Common Chemicals
(calculated for a 160-lb. human from data on rats)
Chemical
Lethal Dose
Sugar (sucrose)
3 quarts
Alcohol (ethyl alcohol)
3 quarts
Salt (sodium chloride)
1 quart
Herbicide (2,4-D)
1/2 cup
Arsenic (arsenic acid)
1–2 teaspoons
Nicotine
1/2 teaspoon
Food poison (botulism)
microscopic
Source: Marczewski, A.E., and Kamrin, M. Toxicology for the citizen. Retrieved August 17, 2000, from http://www.iet.msu.edu/Tox_for_Public/citizen.htm.
• A chemical is considered toxic if it produces adverse effects in a living organism at levels of exposure that are likely to occur. These adverse
effects can range from slight symptoms, such as headache, nausea, or
rashes, to severe symptoms, such as coma, convulsions, and death.
Toxicologists recognize that for most types of toxic responses to a
chemical, there exists a dose threshold below which no toxicity is evident. As the dose increases, more severe toxic responses occur.
Toxicity Testing
How does a toxicologist know when a chemical is toxic to humans? When
available, toxicologists study data from human populations that have been exposed to specific chemicals. The data usually come from studies of
workplace exposure or incidents such as the Union Carbide chemical plant
accident in India. In this way, toxicologists further their understanding of the effects of chemicals on humans. In the absence of human data,
toxicologists test the toxicity of different doses of chemicals on cell and 42
tissue cultures, plants, and other animals, such as rats and mice. These
studies guide toxicologists in their understanding of which chemicals might be harmful to humans and in what amounts.
The use of animals in toxicology research is not taken lightly. Following is the Society of Toxicology’s Animals in Research Policy Statement on the Society of Toxicology’s Web site ( http://www.toxicology.org):
• Research involving laboratory animals is necessary to ensure and
enhance human and animal health and protection of the environment.
• In the absence of human data, research with experimental animals is the most reliable means of detecting important toxic properties of chemical
substances and for estimating risks to human and environmental health.
• Research animals must be used in a responsible manner.
• Scientifically valid research designed to reduce, refine, or replace the need for laboratory animals is encouraged.
Toxicologists know that the kinds of questions they want to answer cannot always be answered by observing and describing humans exposed to
chemicals. They need to devise experiments that involve a system that
resembles the human system. By studying a model of the human system,
rats or mice, for example, toxicologists hope to apply the knowledge
they gain to understanding the harmful effects of chemicals on humans.
Although the basic tenet of toxicological studies is that “experimental
results in animals, when properly qualified, are applicable to humans,”
toxicologists recognize that different species can respond to doses of toxic substances differently (Klassen, 2008). For example, on the basis of dose per unit of body surface, toxic effects in humans are usually about the same as for experimental animals; on a body-weight basis, though, humans are
about 10 times more vulnerable than small experimental animals, such as
mice (Klassen, 2008).
Because of both practicality and ethics, scientists who use animals in
research carefully select the species and design experiments to achieve
scientifically valid results. They obey strict regulations about the use of animals in experiments (Society of Toxicology, 2012). Typically in these
experiments, toxicologists expose experimental animals to high doses of
toxic agents so that they minimize the number of animals they use. This
experimental design assumes that the results of tests at high doses on a
small number of animals can be extrapolated to estimate the risk of low
doses to a large population of humans.
Toxicity testing is not designed to demonstrate that a chemical is safe
for humans, but is used to identify the types of toxic effects a chemical can produce. One early test performed on a chemical is the Ames test,
named after Bruce Ames of the University of California–Berkeley. In
this test, specially engineered bacteria are exposed to a chemical. If the bacteria mutate, the chemical reacted with DNA and is a potential mutagen or carcinogen. Scientists use the Ames test to economically weed out
mutagenic chemicals because it avoids testing on higher animals.
43
Student Lesson 2
Chemicals, the Environment, and You
Often, scientists use cell cultures in toxicology testing. Scientists expose isolated cells to a chemical and observe the response. If the cells die during the experiment, the chemical may be too toxic for use by humans. As
with the Ames test, tests on cell cultures help scientists narrow the list of chemicals they need to test further on animals by eliminating those that are clearly too toxic.
If these preliminary tests suggest a chemical might be used safely with
humans, scientists consider testing with animals. One of the first animal tests that scientists perform on a new chemical determines its acute
toxicity. Toxicologists determine what dose of the chemical, under the
intended route of exposure, causes 50 percent of the animals (mice or rats) to die (lethal dose, or LD ). Toxicologists also determine the effective
50
concentration at which 50 percent of the animals exhibit a measurable
response (EC ).
50
Scientists perform subacute toxicity tests to learn about the toxicity of a chemical after repeated doses. To test a chemical that is likely to enter the body through ingestion, scientists add doses of a chemical (high, low, and intermediate) to the feed for the experimental animals, usually rats or mice.
Each animal receives a specified dose over the course of 90 days. Scientists observe the animals once or twice daily for signs of toxicity, including
changes in body weight, diet consumption, changes in fur color or texture, respiratory or cardiovascular distress, motor and behavioral abnormalities, or palpable masses. They record premature death and collect blood and
tissue samples from all animals for further study. If the chemical is likely to pose a risk to humans through skin contact or inhalation, scientists perform tests that incorporate those routes of exposure. They conduct long-term
or chronic exposure studies in a similar manner, but the exposure time is increased to a time period that can range from six months to two years.
Efforts are under way by at least two groups to reduce the use of animals in some kinds of toxicity testing (NIEHS, 2012 and 2009). For example,
researchers have developed a collagen matrix barrier that serves as a kind of artificial skin. If a chemical or chemical mixture penetrates the artificial skin, it is likely to irritate, corrode, or burn human skin.
For example, in the illustration on page 45, the drawing shows how a
chemical is tested using the collagen matrix barrier. A sample of the
test chemical is dropped onto the matrix. If no chemical penetrates the
matrix, the solution in the bottle below the matrix remains clear. If the chemical penetrates the matrix, it will cause a color change in the solution in the bottle below. The photo on the right shows the indicator solution
changing color after the test chemical has penetrated the matrix. This
method, using artificial skin, can replace the current practice of using three animals to test every new chemical. Because more than 2,000 chemicals are introduced each year and many are tested before they are introduced on the market, this replacement means a significant reduction in the number of
experimental animals used in toxicity testing (NIEHS, 2012).
44
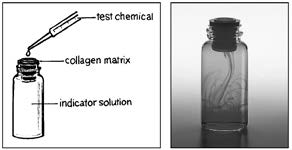
Photo: Courtesy InVitro International
Researchers are also developing techniques that are more accurate than
the traditional methods. In the past, when researchers wanted to know if a blood pressure medicine was working in an animal, they inserted a catheter into an artery in the animal’s leg. The animal then needed to be restrained so that scientists could take readings over a four- to five-hour period. Today, a sensor implanted in the animal’s abdominal cavity allows researchers to continually measure results while the animal can move freely and remain
with its family. Its heart rate is more relaxed and normal, so the results do not mirror the compounding effect of stress.
The rate at which new technology is being used to help researchers
reduce their reliance on laboratory animals is accelerating. People who are concerned about animal welfare are working with researchers to encourage
better experimental design and more humane techniques. Together, they
are working to replace laboratory animals with scientifically valid
alternatives, reduce their numbers, and refine techniques to minimize
pain and suffering.
Even as progress is made in the name of animal welfare, however,
conflicting pressures arise from the public’s interest in knowing more
about the health and safety data on major industrial chemicals. For
example, in October 1998, Vice President Al Gore announced his plan
to collect data on 2,800 high-production-volume chemicals. Animal
rights groups recognized that such testing would require the destruction
of more than 1 million animals. For a year, to minimize the number of
animals and avoid needless testing, animal welfare activists lobbied to
halt or modify the plan. One year later, the U.S. Environmental Protection Agency (EPA) made new recommendations for high-production-volume
chemical testing that should reduce animal use. They now will consider
previous results from chemical safety databases to ensure that testing is not redundant and will postpone the testing of some chemicals in the
hope that nonanimal tests will become available.
45
Student Lesson 2
Chemicals, the Environment, and You
Notes about Lesson 2
In this lesson, students perform toxicity tests on seeds, paying careful
attention to the dose and concentration of chemicals. Students might not be aware that plants differ from animals in many ways: They have no nervous
system or efficient circulatory system, and they have a photosynthetic
mechanism and cell walls that animals do not. Therefore, the students’
results from toxicity tests on seeds cannot be extrapolated to suggest a
chemical’s risk or safety to humans without further testing on animal
systems, which is inappropriate for the classroom. However, students
can understand the importance of using model systems in science when
human subjects cannot be used because of the potential risk. Students can understand that many questions in science suggest a variety of investigation methods and that their use of models in scientific inquiry can help them
establish relationships based on evidence from their own observations.
In Advance
Web-Based Activities
Activities 2 and 4 have optional Web components.
Materials and Preparation
Photocopies and Transparencies
1 transparency of Master 2.1
1 copy of Master 2.2 for each team
1 copy of Master 2.3 for each student (print version only)
Note: If you want students to calculate percent concentration on their own, in Master 2.2, mask the numbers in the concentration column before copying.
Materials
Activity 1
For the class:
• overhead projector
• shoe box from Lesson 1
• 1 small jar containing the mystery chemical from Lesson 1
• 1 eyedropper
• 1 pair of safety glasses
• 1 pair of latex gloves
• 3 1,000-mL beakers or 3 large jars of the same size, each containing 500
mL of water
• 1 piece of white poster board to use as a backdrop for the demonstration
• 1 resealable plastic sandwich bag containing radish seeds
• 1 beaker containing 250 mL of water (optional)
For each student:
• science notebook
46
Activity 2
For the class:
• computer
• chemicals from Lesson 1
• mystery chemical from Lesson 1
• 1 resealable plastic sandwich bag containing radish seeds
For each student:
• science notebook
Activity 3
For each team of 3 students (print version):
• 3 pairs of safety glasses
• 3 pairs of latex gloves
• 1 100-mL beaker filled with 50 mL of a chemical; see Preparation for Activity 3
• 1 permanent marker
• length of masking tape
• 6 50-mL beakers
• 1 50-mL graduated cylinder
• 1 10-mL graduated cylinder
• 100 mL of purified water in a beaker
• 1 eyedropper
• 6 resealable plastic sandwich bags
• 12 paper napkins
• 60 radish seeds in a resealable plastic sandwich bag
• 1 tray
For each student:
• science notebook
Activity 4
For each team of 3 students:
• bags of seeds treated with chemicals from Activity 3 (print version only)
• 1 copy of Master 2.3 from Activity 3 (print version only)
• Web version of data for Day 2 (optional; see Preparation for Activity 4) For each student:
• science notebook
Extension Activity
For the class:
• computers with Web access
• materials for designing a bulletin board display
47
Student Lesson 2
Chemicals, the Environment, and You
Notes on Materials:
1. The mystery chemical is the solution of blue food coloring and water used in Lesson 1.
2. Check that no students are allergic to latex. If any are, assign their team a chemical that you know will not irritate the skin, such as
sugar water or cola. The team members with the latex allergy then
can work safely without gloves. Alternatively, they can use vinyl
gloves, if available.
3. Instead of 50-mL beakers, you could use 6 clean baby food jars or
6 test tubes set up in a rack made out of a shoe box.
4. For the paper napkins, use regular, white, one-ply napkins
(12 x 115/8 inches, unfolded) that you can buy in bulk at the
grocery store. If you use something different, test your setup to
make sure that the napkins or paper towels you use can absorb
20 mL of liquid in a plastic bag.
Preparation
Activity 1
Pour 500 mL of water into each of the 3 1,000-mL beakers. Label the
beakers #1, #2, and #3.
Put a handful of radish seeds in a resealable plastic sandwich bag. Gather the materials you need for the demonstration.
Make a transparency of Master 2.1, Opening Questions.
Activity 2
Gather the materials you will need for this activity.
Copy Master 2.2, Making Solutions for Toxicity Testing, 1 copy for each team. Set up a computer center at which students can view the Chemicals Web site.
Activity 3
Decide whether you will use the print or Web version of the laboratory
investigation. It is tempting to avoid the preparation and materials that a laboratory investigation requires, but students benefit from conducting a scientific investigation, using tools to gather data, and developing a
hands-on understanding of the use of models in scientific inquiry. The
simulation presented on the Web site enables teachers and students
without access to laboratory equipment to gather data to use in Lesson 3, 48
but it should not replace actual laboratory experience. In addition,
Lesson 2’s laboratory investigation provides students with an opportunity to meet Content Standard A of the National Science Education Standards: All students should develop abilities necessary to do scientific inquiry and understandings about scientific inquiry (NRC, 1996).
For the print version (preferred) of the laboratory investigation:
Prepare the chemicals, 1 chemical for each team of 3 students:
1-p. Choose a wide variety of chemicals for testing:
• water-soluble plant food
• liquid detergent
• soft drink
• instant coffee
• nontoxic environmental cleaner
• tempera paints
• all-purpose disinfectant cleaner (Lysol)
• artificial sweetener
• shampoo
• window cleaner
• salt
• sugar
• fruit and vegetable rinse
Tip from the field test: During the field test, the following chemicals yielded data that made the most interesting dose-response curves for
students to graph in Lesson 3: salt, Miracle Gro, fruit punch soft drinks, window cleaner, and Lysol. The results from other chemicals were also
of interest to students, so be sure to include a wide variety of familiar chemicals, such as shampoo, soft drinks, coffee, and sweetener, even if their dose-response curves are less exciting. One of the reasons to use a variety of chemicals is to demonstrate the range of responses that are possible.
2-p. Measure 50 mL of each liquid chemical into a 100-mL beaker.
Label the beaker with the name of the chemical (for example,
window cleaner).
3-p. Make solutions of nonliquid chemicals by mixing them with water.
Then, measure 50 mL of each liquid solution into a 100-mL beaker.
Label the beaker with the name of the chemical.
When available, follow directions on the container to make solutions
of nonliquid chemicals, such as plant food or instant coffee. When no
directions are available, make as saturated a solution as possible: Heat
the water and slowly stir in a small amount of the chemical until it no
longer dissolves easily in the water. In pilot testing this activity, we
made a sugar solution with 40 g of sugar in 100 mL of water and a salt
solution with 24 g of salt in 100 mL of water. Be sure to make enough
solution for all your classes.
49
Student Lesson 2
Chemicals, the Environment, and You
4-p. Place each chemical on a tray, 1 tray and chemical per team.
Purchase radish seeds. Put 60 radish seeds into a resealable
plastic sandwich bag. Continue until you have a bag for each team
of 3 students.
Radish seeds found in a local garden store work well for this investigation.
They will germinate in 1 to 3 days. If you prefer faster germination
(6–24 hours), you can purchase Wisconsin Fast Plants™, Brassica rapa seeds (which are close relatives of the radish) from Carolina Biological Supply. Be aware that the Brassica rapa seeds are quite a bit smaller than radish seeds, so consider your students’ dexterity when deciding which seeds to use.
Tip from the field test: Counting the 60 seeds can be time consuming.
Estimate the number of seeds by measuring approximately ¼ teaspoon of
regular radish seeds (less if you use Brassica rapa seeds) for each bag. There will be a little more than 60 seeds in each bag. Students tend to lose a few as they set up the investigation, so it doesn’t hurt to have a few extra seeds in the bag or on hand at the materials table.
Activity 4
If your students conducted the Web version of Activity 3, arrange for them to have access to computers for this activity.
Extension Activity
Arrange for students to ha




