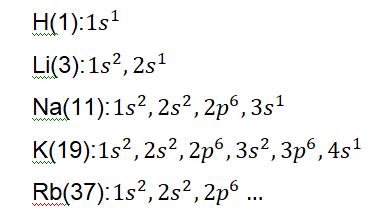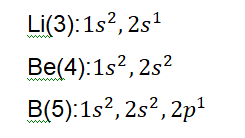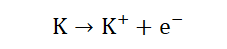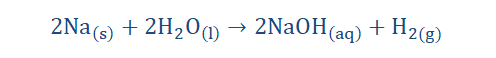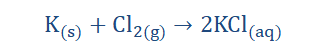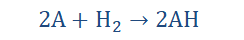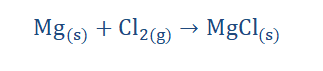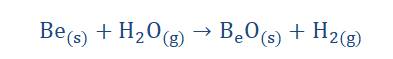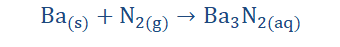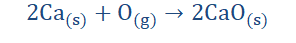CHAPTER 9: THE PERIODIC TABLE
Groups and Periods
Well, hello again. We have been talking extensively about various elements on the periodic table but we haven’t paused to consider how these elements occur and in what ways they relate to one another. In understanding these, the world of chemistry
becomes crystal clear. In understanding the periodic table we begin to see how and why elements and compounds behave in the ways they behave. We study not just the
physical attributes of these elements but their chemical characteristics as well. Enough talking, let’s get right on the task. Below is the periodic table once more to aid us in our discourse.
I bet that being a sharp thinking person you must have at some point in our discussion wondered about the arrangement of the table. Are the elements placed at random
locations or is there some method to the madness of this table of letters and numbers?
Even though chemistry seems like madness, there are always precise reasons for all that we do as is characteristic of all sciences. For instance if you look up at the table you find that there are rows and columns. The rows move from one side to the next horizontally on the page, while the columns go up and down vertically on the page. The horizontal arrangement constitutes the periods while the vertical arrangement makes 201

the groups of the periodic table. Don’t be fooled by the seeming simplicity of this table that looks more like a calendar because a lot of interesting things are going on here.
“The periodic table is an organized arrangement of
chemical elements in rows and columns on account
of increasing atomic numbers.”
This definition definitely brings something else to mind to consider and that is the concept of atomic numbers.
Figure 9-1Dimitri Mendeleev
I know what you’re thinking and even though we don’t have to say it my big mouth will do just so-That is an intimidating looking man. Yes, yes he is but do not be carried away by how he makes you want to confess all your sins; be carried away instead by his brilliant and yes, intimidating mind for the man in the picture is none other than Dimitri Mendeleev the ‘father’ of the periodic table.
He didn’t somehow birth the periodic table into existence the way we imagine
reproduction in biological terms, but he did bring order into the disordered array of the grouping of elements. Being a renowned lecturer in Russia, he felt it his duty to write a chemistry text just like I am writing one except in his case he hadmore pressing reasons to (there were seldom any modern organic chemistry textbooks in Russian and the disordered elements gave him a headache). We can very easily picture him sitting at his desk, grouping and regrouping the elements like a weird jigsaw puzzle. We see him suddenly get up. Yes, that’s an ‘aha!’ moment as he discovers that when grouped 202
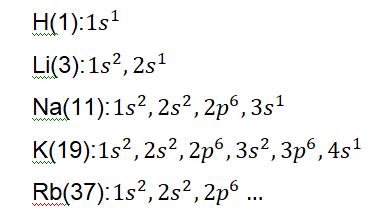
according to their atomic numbers the elements begin to show an interesting pattern and now he could even predict certain elements that have not even been discovered yet.
The Groups and the Periods
We have gone over a brief description of what groups and periods are. Now turn to the periodic table and look at it once more. What element begins the first group? I’ll wait.
If you stated hydrogen, you’d be right. Under hydrogen we find Li, Na, K, Rb, Cs and Fr (Look at the table to see what the symbols mean). The second group begins with
Beryllium(Be) then Mg, Ca and so on. You could keep going until you reach the last group that begins with He. Have you observed that elements with the smallest atomic numbers begin the groups and those with larger atomic numbers are found down the
group? Hold on to this observation and let’s move on.
What about periods? H and He stand alone so let’s take a look at the next element Li which starts the second period, followed by Be, then B, C, N, O, F and Ne. If we check period 3 we find Na, Mg and so on. Can you see that the atomic numbers also increase across the period?
Let’s zoom in a little on what’s going on in the background. We have learnt about electronic shells and configurations. I am certainly glad we studied that because that would make understanding this a breeze. If we study the group 1 elements we notice something interesting.
We don’t need to finish writing down the configuration for the entire group, as I am quite certain you’ve noticed the pattern here as well. If you look closely, you find that the valence electrons (you recall the meaning) are exactly the same for each element in the same group. Here we see that the number of valence electrons for the elements of
group 1 is 1. If we proceed to writing down the electronic configurations of the elements of group 2 we find a similar pattern. All the elements have 2 valence electrons each.
Why not try writing them down? I’ll wait.
203
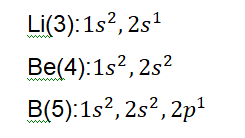
It is important to note that we put hydrogen in group 1 just for the convenient reason that it has a single valence electron. However, in reality H belongs to no particular group. The same can be said for helium (He). While its valance electrons are 2, the other elements of group 8 (or group 0 in some texts) have valence electrons of 8.
I am quite sure you have seen the pattern with your own eyes. We can now safely say that elements in the same group have the same number of valence electrons.
Periods
Now that we have understood what basic factor determines group arrangement let’s
take a look at periods. You must recall what shells are. They dertermine the
arrangement iof elements in groups. For instance elements in period 2 have 2 electron shells (K and L). Elements in period 4 have 4 electron shells(K, L, M, N). How many electron shells do elements in period 3 have?
In addition to shells we also find a pattern with regards their valence electrons.
Notice how the number of valence electrons increases by one with each element? Li has 1, Be has 2 and B has 3; yes, 3. Don’t be confused by the number ‘1’ hanging
above ‘2p’. The number of valence electrons is actually a combination of them meaning 3.
How Atomic Properties Become Clearer with the Periodic Table
Just like people have different properties that make them who they are, atoms are different too. Just like you have people of different shapes and sizes, atoms have different shapes and sizes too and just as people react differently to things, atoms are different when placed in certain conditions. These differences are also clearly seen when we look at the periodic table. Mendeleev did that in his groupings and we are going to take a look at them too.
Sizes (Atomic Radii)
We have already mentioned that the number of valence electrons increase as we move down the group of the periodic table. This could also translate to saying that the number of valence shells increase as well. It just makes sense. Imagine having a birthday party for twelve people. It is logical that you set out twelve seats to accommodate the twelve people. If however you realize that more people would be attending your party you set out more seats depending on the number of people that would be coming. So the
204
number of seats increases with the number of guests expected. The same goes for the group elements. As the number of electrons increases down the group, the number of valence shells increases so as to accommodate the increasing number of electrons. In the end we find that the atomic size increases as well so that the last element in the group would be larger than the first. Knowing this, it is now no surprise that the atomic size of Radium (Ra) is larger than Beryllium(Be).
If you understood what was just explained we can proceed to explain atomic and ionic sizes in the context of electron clouds (which is a more modern take on atomic structure which we have previously examined).
Unlike the neat little mental picture we painted with valence shells, electron clouds are little more difficult to picture. It is difficult to come to terms with their specific sizes but we take into account relative distances between nuclei using x-ray diffraction studies.
The same can be said for ionic sizes which are just atomic sizes that undergo changes due to electron transfer i.e. when the atom loses or gains electrons. Down the group the electron cloud enlarges due to a reduction in the force of attraction between the positive charge of the nucleus and the electrons farther from it. The increasing number of shells however does not allow for a reduction in atomic size since they are farther away from the positive charge.
For the period however, the sizes of the atoms decreases progressively as we move from one element to the other from left to right. This is because the increasing number of electrons means an increase in the positive charge. This in turn means that the positive charge pulls in the electrons to itself even more allowing for a more ‘compact’
atom size. Unlike groups that extra shells are added, the number of shells across the period remains the same.
Electronegativity
We have discussed electronegativity before now but there’s of course no crime in briefly having another look. You recall that electronegativity is the tendency of an atom of an element to accept electrons. Certain atoms such as Na have only one electron on their valence shells and hence they tend to give electrons and not accept. Na is therefore not electronegative, but electropositive because it easily gives the electron more than it accepts electrons. An atom of Fluorine however has 7 electrons on its valence shell and hence needs only 1 electron to complete the octet configuration and attain stability. F2
can therefore be said to be electronegative. In the periodic table, electronegativity tends 205

to decrease as you go down the group due to the fact that atomic radii increases. Can you explain why this is so? I’ll wait.
Figure 9-2 I chuckled a little
If you have been following (and I believe you have), you would understand that as the atomic radii increases down the group, there is more space on the shells for electrons.
Lower number of electrons on the valence shells means that the corresponding atom would be quite far from an octet configuration, and hence more willing to lose electrons to reach that configuration, than accept electrons on its shells.
But what happens to electronegativity across the period? Well it increases across the period from left to right. This happens because the atomic sizes decrease as we
proceed in that direction. In this case you have more electrons present on an average valence shell as you move from left to right. These usually need about one or two electrons to complete the octet configuration and hence are willing to accept electrons.
Electron Affinity
Electron affinity generally means the readiness of an atom or other elementary particle to accept electrons. Don’t be confused by thinking electronegativity and electron affinity are the same thing. While electronegativity is the tendency to accept electrons, electron affinity is the energy released when an electron is added to an atom to form an ion (an anion). From this definition it is quite possible for you to guess what the periodic table trend is for this. Did you come to see that it must therefore increase across the period? Since more electrons are on the valence shells, the tendency to accept
electrons would increase from left to right as more atoms would need one or two
electrons to complete their octet configurations. It also largely decreases down the group for reasons I am certain you are aware of now.
206
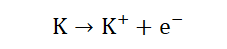
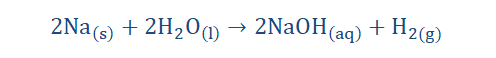
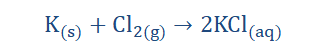
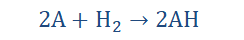
THE GROUPS
Group 1(Alkali Metals)
Chemical Properties
1. Due to their single valence electrons they show similar chemical reactions.
2. This single valence electron makes them highly electropositive (able to lose
electrons) and hence very reactive.
3. Their reactivity however differs, as elements down the group are more reactive than preceding elements. This is because as we proceed down the group the
number of shells increases making the valence electrons move further from the
nuclear charge and hence readily able to be removed. In addition to this, the
screening effect of inner electrons allows for further distancing of valence electrons from the nuclear charge. This screening effect occurs when
surrounding electrons repel each other due to having the same charge
(negative). This makes the attractive force between the electrons and the nuclear charge weakens, making it easy for them to be removed from the shells; hence
reactivity is said to increase down the group.
4. Since reducing agents give out electrons readily, it is safe to say that group 1
elements are good reducing agents. For the same reasons described above,
their strength as reducing agents increases as we go down the group.
5. They readily react with water to form metal hydroxides and H.
6. They react with chlorine (Cl) to form metal chlorides.
7. They generally react with group 7 elements also known as the halogens (we’re getting there) to form metal halides. The general form for this reaction is;
Where A: group 1 element and H is the halide, making AH the metal halide
formed as a product of the reaction. Note that chlorine is a halide as well.
207

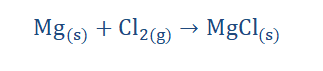
Physical Properties
1. They are usually referred to as soft solids as they are malleable and can be cut with ease.
2. They can conduct heat and electricity easily.
3. They don’t seem to react well to the open air unlike you and I (perhaps) because they turn dull when exposed to air.
4. They have low boiling and melting points.
5. They are grey solids and can appear silvery when cut.
6. Their densities are comparably low compared to other metals.
Figure 9-3 Na; a group 1 element
Group 2(Alkali-Earth Metals)
Chemical Properties
1. They react with the halogens to form metal halides, just like the group 1 metals do.
208
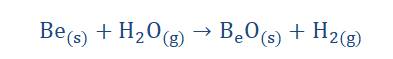
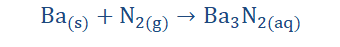
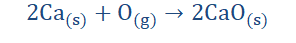

2. On reacting with water, they tend to produce metal hydroxides and evolve
hydrogen gas. Be however does not follow this trend as it does not react with
water.
3. They could react with Nitrogen to produce nitrides. This reaction must occur in conditions of high temperature.
4. When they react with oxygen, they form oxides.
Physical Properties
1. Even though they appear ‘soft’. They are harder than group 1 metals. However
don’t go biting down on either of them if you value your life.
2. They are electropositive and this increases down the group.
3. They are highly conductive both thermally and electrically (please don’t bite down on them).
4. They have higher melting and boiling points in comparison to the alkali metals.
Figure 9-4Beryllium metal
The Transition Metals
The transition metals are the elements that exist in the upper blocks of groups 3 to 12.
It would be helpful to note that these elements are not the lower separated blocks under the groups, but the higher ones. This would become clearer in time so don’t fret.
Properties
1. They have high boiling points
209

2. They can be very hard
3. They can be used as good catalysts
4. They show metallic luster
5. They can be very malleable (arts and crafts anyone?)
6. They typically have low ionization energies
7. They conduct electricity well
8. They have high melting point
Figure 9-5 Transition crafts anyone?
Group 13
Properties
1. They have three valence electrons in their outermost shells.
2. They form electrovalent/ionic compounds
3. They form amphoteric oxides and hydroxides (both acidic and basic properties) 4. They are softer and hence more malleable (does this remind you of another
group?)
5. They have low melting points
6. They are not very conductive
210
Figure 9-6Gallium melts in your hands
Group 14
Properties
1. They are tetravalent; meaning they have four valence electrons (‘tetra’ meaning four.)
2. Reactivity decreases down the group.
3. C, Si and Gm are not affected by diluted acids and water
4. Sn and Pb are amphoteric in nature.
5. Even though Pb and Sn are less reactive than the others, they are still able to react with the halogens (group 17 elements).
6. There are two oxidation states present in this group. While the +2 oxidation state increases as we move down the group, the +4 oxidation state decreases as we
proceed down the group from C to Sn.
211

Figure 9-7Carbon is quite an interesting element
Group 15
Properties
1. The density of the elements increase as we move down the group. N for instance is a gas, in the middle of the group we find metalloids such as As and then finally we find metals such as Bi.
2. There is an increase in atomic and ionic radii as we move down the group for
reasons earlier explained. However, the increase from As to Bi is only relatively slight. This happens because of the presence of completely filled f or d orbitals in the more dense elements.
3. Since the atomic size increases as we slide down the group, electronegativity naturally decreases and we both know why.
4. The ionization energy as we move down also decreases since the atomic radii
increases. If you remember clearly what ionization energy is you know that this has something to do with the nucleus having a weaker hold on the electrons.
5. There is an increase in boiling point down the group.
212

6. Allotropes of the elements exist except for N. (Allotropy is the ability of an element to exist in different physical forms but in the same chemical state. Water for instance has the gaseous (steam), liquid and solid (ice) allotropic forms while still having the same chemical state (two hydrogen atoms, one oxygen atom per
molecule))
Figure 9-8Antimony is an interesting name
Group 16
Properties
1. They are also known as chalcogens.
2. They are electropositive
3. Ionization energy and electron affinity decrease as we move down the group.
4. The atomic radii increase down the group
5. Metallic character increases down the group
6. Melting and boiling points increase down the group with increasing atomic
numbers
213

Figure 9-9Mined Sulphur
Group 17
Properties
1. These are known as the halogens or salt formers
2. They have seven valence electrons in their valence shells
3. They are coloured; the colours become darker as we go down the group, with F2
being pale yellow, white or even colourless and I2 being blue/black.
4. Their molecules are diatomic
5. They have low melting and boiling points due to weak van der waals forces
holding their atoms together.
6. They are weak conductors of heat and electricity
7. They have low densities and hence are all non-metals.
8. Their reactivity decreases as we go down the group so that Cl2 is more reactive than Br2 which is more reactive than I2and I2 more than At2 (you get the picture).
9. They are highly electronegative
10. There is a gradation in their states as F2 and Cl2 are gaseous, Br2 is a liquid and I2 and At2are solids at room temperature.
214

Figure 9-10Astatine
Group 18
Properties
1. These are the rare or noble gases (picture a serene unreactive yoda floating in midair as a visual aid)
2. They have no colour, odor or taste (yum)
3. Their atomic radii increase as we go down the group
4. They are gasses at room temperature
5. They generally have low melting and boiling points and their melting and boiling points increase down the group.
6. They have low densities and are insoluble in water
7. They do not conduct electricity and conduct heat only a little.
8. Noble gasses have a duplet configuration and this allows them to be very stable 9. They are inert in that they do not engage in reactions by moving their electrons about (in accepting, giving away or sharing). This makes them unreactive.
10. They are generally non-flammable.
215

Figure 9-11 Helium is used to fill balloons of all shapes and sizes
Lanthanides and Actinides
Lanthanides
Properties
1. These elements are the first of the two separated blocks of elements below the transition elements that are situated between groups 4 and 18 on the periodic
table.
2. The first element from left to right is lanthanum and since they have similar properties they are all referred to as lanthanides.
3. They are generally silver coloured.
216

4. They are very reactive elements.
5. They are also known as rare earths.
6. Their atomic numbers varies from 57 to about 71.
7. Their densities increase down the group. They can be relatively soft though.
8. They have high melting and boiling points
9. They can burn in air fairly easily
10. They are good reducing agents.
Figure 9-12 Cerium
Actinides
Properties
1. They are called Acting elements and range in atomic number from 89 to 103.
2. They are located in the last row below the lanthanides; the first element from left to right is actinium and their similar properties have given them the name actinides.
3. Their isotopes are unstable and they are all radioactive so it’s safe to treat them with caution.
4. They spontaneously ignite in air hence they are said to be pyrophoric.
5. Like the lanthanides, they are also quite soft. This makes them easily malleable.
6. They are very electropositive.
217

Figure 9-13 Plutonium
It is good to note that the lanthanides and actinides are often referred to as the inner transition elements. They seem to be the odd ones out and are separated from the rest of the family because they just can’t seem to quite fit in. If they did fit in they would make the table unnecessarily difficult and cumbersome (Imagine this table but in 3d) Since we would like to sleep well tonight we would skip the reason for this separation, even though I encourage you to read up on it on your own if your merciful teachers would allow a scheme that could give you a little leg room for that.
218
Well, that was definitely an interesting find. And it’s all thanks to Mendeleev else it would have been majorly difficult to make the many discoveries we continue to make now on account of the periodic table. Remember to give old Mendeleev a little thanks as you sip your drink and prepare to read some summaries and enjoy some practice
questions- the best part
219
SUMMARY
The periodic table is an organized arrangement of chemical elements in rows
and columns on account of increasing atomic numbers.
The elements on the periodic table are divided into groups and periods. Groups consist of the vertical arrangement of elements from top to bottom, while periods include the horizontal arrangement from left to right.
Dimitri Mendeleev, a brilliant Russian lecturer discovered the periodic table of elements.
Factors such as electronegativity, electron affinity and atomic radii (size)determine the arrangement of elements on the periodic table.
There are 18 groups in the periodic table (1 to 18, including the transition
elements) though some texts show groups 1 – 0 (0 being group 18, representing
the noble gasses, not including the transition elements) where we have groups
1,2,3,4,5,6 and 7. However this is an older method and is not largely in use
anymore.
The lanthanides and actinides are heavy elements located at the lower portion of the table.
Group elements have similar characteristics.
220
MNEMONICS
What seems mnemonic worthy today? Look through the text and see if you can find
any. Remember to keep it simple so it’s easy to remember.
221
REVISION QUESTIONS
1. What is the periodic table?
2. Who is considered to be the father of the periodic table
3. Explain the following with regards to periodic table trends:
a) Electronegativity
b) Electron Affinity
c) Atomic radii
4. List 5 properties each of the following groups:
a) Group 1
b) Group 2
c) Group 13
d) Group 14
e) Group 15
f) Group 16
g) Group 17
h) Group 18
i) Transition Elements
j) Lanthanides
k) Actinides
5. For group one elements like H, how many valence electrons are on the valence
shell?
222
223
REFERENCES
1. Figure 1-1. Lightening. Adapted from National Geographic Kids, by Mihai Simonia, Retrieved from
https://kids.nationalgeographic.com/science/article/lightning-.
2. Figure 1-2. John Dalton, F.R.S. Adapted from Science History Institute, by William Henry Worthington after an 1814 painting by William Allen, Retrieved from https://www.sciencehistory.org/files/dalton1-profilejpg
3. Figure 1-3. John Dalton S Periodic Tables. Adapted from imagedog, Retrieved from https://imagedog.vercel.app/8/posts/the-best-15-science-symbols-list/
4. Figure 1-4. J.J Thomson: Cathode ray tube. Adapted from Chemistry LibreTexts, credit a) modification of work by Nobel Foundation; credit b) modification of work by Eugen Nesper; credit c: modification of work by “Kurzon”/Wikimedia
Commons. Retrieved from
https://chem.libretexts.org/Courses/University_of_Arkansas_Little_Rock/Chem_1
402%3A_General_Chemistry_1_(Belford)/Text/2%3A_Atoms%2C_Molecules%2
C_and_Ions/2.01%3A_Atoms%3A_Their_Composition_and_Structure
5. Figure 1-5. Thomson’s Plum Pudding or Chocolate Chip Cookie Model of the Atom. Adapted from Flatworld, Retrieved from
https://scholar.flatworldknowledge.com/books/4309/averill_1.0-ch01_s05/preview
6. Figure 1-6. Ernest Rutherford. Adapted from 10 Facts, Retrieved from
https://10factstop.blogspot.com/2017/08/10-facts-about-nuclear-energy.html
7. Figure 1-7. Rutherford Model of the Atom. Adapted from Another Level Higher, Retrieved from https://anotherlevelhigher.wordpress.com/2016/08/25/781/
8. Figure 1-8. Bohr Model. Adapted from QS Study, Retrieved from
https://qsstudy.com/chemistry/bohr-model
9. Figure 1-9. Erwin Schrodinger. Adapted from The Nobel Prize, Retrieved from
https://www.nobelprize.org/prizes/physics/1933/schrodinger/biographical/
10. Figure 1-10. Electron Cloud. Adapted from Atomic Theory Timeline, Retrieved from https://paininmyassproject.weebly.com/erwin-schrodinger.html
11. Figure 1-11. Elementary particles included in the standard model. Adapted from Wikepedia; The Free Encyclopedia, Retrieved from
https://en.wikipedia.org/wiki/Elementary_particle
12. Figure 1-12. Particle Hierarchy. Adapted from Pinterest, Retrieved from
https://www.google.com/imgres?imgurl=https://i.pinimg.com/originals/eb/55/3e/eb
224
553e3564dd171ca9c0899f328ea6d9.gif&imgrefurl=https://www.pinterest.com/po
dgaets/phisics/&h=640&w=250&tbnid=akjAC26NTPGJPM&tbnh=360&tbnw=140
&usg=AI4_-kQcaCyh3BfQCbv04RfwPU0kCFiEQw&vet=1&docid=-
U0IMz_kCrHFKM&itg=1&hl=en_GB
13. Figure 2-2. A Modern Periodic Table. Adapted from Chemistry libreTexts, Retrieved from
https://chem.libretexts.org/Courses/Heartland_Community_College/CHEM_120%
3A_Fundamentals_of_Chemistry/02%3A_Atoms_and_Elements/2.02%3A_Perio
dic_Table
14. Figure 2-3. Broken Seesaw. Adapted from Esoteric Thoughts, Retrieved from
http://catate.blogspot.com/2011/07/unbalanced-relationships-sometimes-
feel.html
15. Figure 3-1(a). Sailing Lakes. Adapted from World Sailing Charters, Retrieved from https://www.worldsailingcharters.com/best-sailing-lakes-in-california/
16. Figure 3-1(b). Beautiful Space Wallpapers. Adapted from Wallpaper Cave, Retrieved from https://wallpapercave.com/beautiful-space-wallpapers
17. Figure 3-1 (c). Angry businesswoman is slapping across the businessman’s face.
Adapted from Shutterstock, by Sergey Peterman, Retrieved from
https://www.shutterstock.com/image-photo/angry-businesswoman-slapping-
across-businessmans-face-73107073
18. Figure 3-2. Gilbert Newton Lewis. Adapted from Berkeley College of Chemistry, Retrieved from https://chemistry.berkeley.edu/news/gilbert-newton-lewis
19. Figure 3-3. Valence Electrons. Adapted from Science ABC, Retrieved from
https://www.scienceabc.com/pure-sciences/how-to-find-the-number-of-valence-
electrons-in-an-element.html
20. Figure 3-4.KLMN Shells. Adapted from Christian Schnell, Retrieved from
https://christian-schnell.info/photo/what-is-k-l-m-n-in-chemistry.htm
21. Figure 3-9. Sodium and Chlorine dot structures. Adapted from Wraptia, Retrieved from https://wraptia.blogspot.com/2021/05/valence-shell-vsepr-definition-
valence.html
22. Figure 3-11. NaCl dissolving in water. Adapted from CHM 101 General Chemistry, Retrieved from
http://www.ltcconline.net/stevenson/2008CHM101Fall/CHM101LectureNotes200
81022.htm
225
23. Figure 3-12. Hydrogen bonds between water molecules. Adapted from Oregon State University, Retrieved from
https://open.oregonstate.education/aandp/chapter/2-2-chemical-bonds/
24. Figure 4-1. Crystalline and Amorphous solids. Adapted from Chemistry LibreTexts, Retrieved from
https://chem.libretexts.org/Bookshelves/General_Chemistry/Map%3A_A_Molecul
ar_Approach_(Tro)/12%3A_Solids_and_Modern_Materials/12.04%3A_The_Fun
damental_Types_of_Crystalline_Solids
25. Figure 4-2. Particles in a liquid. Adapted from BrainStudy, Retrieved from
https://brainstudy.info/images/particles-in-a-liquid
26. Figure 4-3. Gas particles. Adapted from TechnologyUK, Retrieved from
https://www.technologyuk.net/science/matter/states-of-matter.shtml
27. Figure 4-4. Change of State. Adapted from Chemistry Glossary, Retrieved from
https://glossary.periodni.com/dictionary.php?en=change+of+state
28. Figure 4-5. Plasma state. Adapted from GuyHowto, Retrieved from
https://www.guyhowto.com/plasma-state/
29. Figure 4-6 (a). SatyendraNath Bose. Adapted from New World Encyclopedia, Retrieved from
https://www.newworldencyclopedia.org/entry/Satyendra_Nath_Bose
30. Figure 4-6 (b). Albert Einstein. Adapted from biography.com, Retrieved from
https://www.biography.com/scientist/albert-einstein
31. Figure 4-7. Bose Einstein condensate simulation. Adapted from Science News, Retrieved from https://www.sciencenews.org/article/50-years-ago-millionth-
degree-above-absolute-zero-seemed-cold
32. Figure 4-8. Five States of matter. Adapted from Rotary club of Balclutha, Retrieved from
https://www.google.com/imgres?imgurl=https%3A%2F%2Fclubrunner.blob.core.
windows.net%2F00000013188%2FImages%2Ffive-states-of-
matter.jpg&imgrefurl=https%3A%2F%2Fbalclutharotary.club%2Fstories%2Fquan
tum-world-with-dr-eyal-
schwartz&tbnid=zx_rZQVT2dj58M&vet=12ahUKEwiT6IzEsbDxAhUIXxoKHX8RA
eoQMygAegQIARAf..i&docid=a-
n1hA3uqsc4RM&w=728&h=546&q=all%205%20states%20of%20matter&hl=en_
GB&ved=2ahUKEwiT6IzEsbDxAhUIXxoKHX8RAeoQMygAegQIARAf
226
33. Figure 4-9. Boyle’s law. Adapted from BC Campus, Retrieved from
https://opentextbc.ca/anatomyandphysiologyopenstax/chapter/the-process-of-
breathing/
34. Figure 4-10. Charles’ law. Adapted from ChemsitryLibreTexts, Retrieved from
https://chem.libretexts.org/Bookshelves/General_Chemistry/Book%3A_General_
Chemistry_Supplement_(Eames)/Gases/Gas_Laws
35. Figure 4-11. Gay Lussac’s law. Adapted from Let’s Talk Science, Retrieved from
https://letstalkscience.ca/educational-resources/backgrounders/charles-law-and-
gay-lussacs-law
36. Figure 4-12. Pressure. Adapted from Shutterstock, by Tatyana Dzemileva,Retrieved from https://www.shutterstock.com/image-photo/tired-
mother-kids-girl-jumping-on-515256517
37. Figure 4-13. William Henry. Adapted from World of Chemicals, Retrieved from
https://www.worldofchemicals.com/82/chemistry-articles/william-henry-developer-
of-henrys-law.html
38. Figure 5-1. Group 1- The Alkali metals. Adapted from Chemistry LibreTexts, Retrieved from
https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Book%3A_Chemist
ry_of_the_Main_Group_Elements_(Barron)
39. Figure 5-2. Catalysts. Adapted from Rader’s Chem4Kids, Retrieved from
http://www.chem4kids.com/files/react_catalyst.html
40. Figure 5-5. Burning forest. Adapted from Clark Fork Coalition, Retrieved from
https://clarkfork.org/summer-weather/
41. Figure 5-6. Formation of precipitate. Adapted from Quizizz, Retrieved from
https://quizizz.com/admin/quiz/60805239bf91d6001b06e7ee/characteristics-of-
chemical-reactions-flashcard
42. Figure 5-7. Redox reactions. Adapted from The Fact Factor, Retrieved from
https://thefactfactor.com/facts/pure_science/chemistry/physical-chemistry/redox-
reactions/11959/
43. Figure 5-8. Redox. Adapted from Shutterstock, by OSweetNature, Retrieved from
https://www.shutterstock.com/image-vector/oxidation-reduction-loss-gain-
electrons-compounds-1263276619
44. Figure 5-9. Oxidation states. Adapted from Ontola, Retrieved from
https://www.ontola.com/cs/ondi/wlwmgs/proc-ma-ethanol-oba-uhliky-s-oxidacnim
227
45. Figure 5-10. Corrosion, steel, iron, old, industry, rust. Adapted from Pixnio, by Ulleo, Retrieved from https://pixnio.com/objects/corrosion-steel-iron-old-industry-
mechanism-rust
46. Figure 5-11. Acids and bases. Adapted from Pinterest, Retrieved from
https://www.pinterest.com/pin/344736546462740328/
47. Figure 5-12. Ronda Rousey and Miesha Tate. Adapted from gettyimages, by Josh Hedges, Retrieved from https://www.gettyimages.com/detail/news-photo/ronda-
rousey-throws-miesha-tate-in-their-ufc-womens-news-
photo/459660287?adppopup=true
48. Figure 5-13. Collision Theory. Adapted from SlideToDoc, Retrieved from
https://slidetodoc.com/collision-theory-collision-theory-what-is-necessary-for/
49. Figure 6-1. 400 meters relay race. Adapted from Wikimedia Commons, by Arch-Angel Raphael the Artist, Retrieved from
https://commons.wikimedia.org/wiki/File:400_meters_relay_race.jpg
50. Figure 6-2. Exothermic and endothermic. Adapted from exo 2020, Retrieved from
https://exo2020reborn.blogspot.com/2020/02/exo-and-endothermic.html
51. Figure 6-3. Born-Haber. Adapted from English tenses, Retrieved from
https://www.google.com/imgres?imgurl=https%3A%2F%2F1.bp.blogspot.com%2
F-
nnR3BveSImE%2FWEF9D7st0_I%2FAAAAAAAACd8%2FLVOFbS4UjKwBC6C
Mac7ecW8ww9FJdsCPQCLcB%2Fs1600%2Fborn-haber-cycle-of-
nacl.png&imgrefurl=https%3A%2F%2Fenglishtenses.pro%2Fphotos%2Fhow-to-
find-lattice-energy-born-haber-cycle&tbnid=1qDXzb-
s_a5CbM&vet=12ahUKEwjggdjp_bDxAhUF_BoKHQ7pDTgQMygAegQIARAf..i&
docid=hVb0U-iA--
tjbM&w=467&h=391&q=born%20haber%20cycle%20sodium%20iodide&hl=en-
NG&ved=2ahUKEwjggdjp_bDxAhUF_BoKHQ7pDTgQMygAegQIARAf
52. Figure 6-4. Space explosion. Adapted from Motion Array, by RajPakhare, Retrieved from https://motionarray.com/stock-motion-graphics/space-explosion-
35930/
53. Figure 7-1 (a). Balanced seesaw. Adapted from burnabyschools, Retrieved from
https://learning.burnabyschools.ca/wp-content/uploads/2021/01/Science-5-Feb-
1-5_Contact-and-Non-Contact-Forces.pdf
228
54. Figure 7-1 (b). Passing (juggling). Adapted from Wikipedia, The Free Encyclopedia, Retrieved from https://en.wikipedia.org/wiki/Passing_(juggling)
55. Figure 8-1. Faraday’s Electrolysis Experiment. Adapted from FineartAmerica, by Sheila Terry, 1833, Retrieved from https://fineartamerica.com/featured/faradays-
electrolysis-experiment-1833-sheila-terry.html
56. Figure 8-2. Electrolysis. Adapted from Quizizz, Retrieved from
https://quizizz.com/admin/quiz/60abd67d582c75001bdc7dab/electrolysis-of-
copper-sulphate
57. Figure 8-3. Electrolysis of Copper sulphate. Adapted from Electronics Easy Top, Retrieved from https://www.electronicafacil.top/bateria-plomo-acido/principio-de-
la-electrolisis-del-electrolito-de-sulfato-de-cobre/
58. Figure 8-4. Galvanic Cell. Adapted from Wikipedia, The Free Encyclopedia, Retrieved from https://en.wikipedia.org/wiki/Galvanic_cell
59. Figure 8-5. Svante Arrhenius. Adapted from Sutori, Retrieved from
https://www.sutori.com/story/global-warming-timeline--
shaD5HmXb75JGifZvapA7Q35
60. Figure 8-6. The Electrochemical Series. Adapted from Gate Academy, Retrieved from https://gateacademy.com.ng/study-by-topics/senior-school-
study/chemistry/electrolysis/
61. Figure 9-1. Dimitri Mendeleev. Adapted from Wikipedia, The Free Encyclopedia, Retrieved from
https://www.google.com/imgres?imgurl=https://upload.wikimedia.org/wikipedia/co
mmons/thumb/8/8f/Dmitri_Mendeleev_1890s.jpg/150px-
Dmitri_Mendeleev_1890s.jpg&imgrefurl=https://en.wikipedia.org/wiki/Periodic_ta
ble&h=191&w=150&tbnid=Kb0mmSJit3-bOM&tbnh=191&tbnw=150&usg=AI4_-
kTeJ3pRTdfIt97A1G7Kw7Zyb0UASg&vet=1&docid=09cCjaH0MxNCvM&itg=1&h
l=en
62. Figure 9-2. Mendeleev. Adapted from Sweatpants and Coffee, Retrieved from
https://sweatpantsandcoffee.com/sweatpants-sanity-brain-soothers-periodic-
tables-awesomeness-edition/10-mendeleev/
63. Figure 9-3. Beryllium. Adapted from Chemical Book, Retrieved from
https://m.chemicalbook.com/ChemicalProductProperty_DE_CB4226453.htm
64. Figure 9-4. Manganese. Adapted from ntra.nasa.gov, Retrieved from
file:///C:/Users/HP/Downloads/20160001416.pdf
229
65. Figure 9-5. Gallium. Adapted from YouTube, Retrieved from
https://www.google.com/url?sa=i&url=https%3A%2F%2Fwww.youtube.com%2F
watch%3Fv%3DMke6ZTgVlzw&psig=AOvVaw2x8JoL1dHAZ1xMfOj4MIIw&ust=1
624704699555000&source=images&cd=vfe&ved=0CAsQjhxqFwoTCPCJ3ITPsv
ECFQAAAAAdAAAAABAE
66. Figure 9-6. Coal. Adapted from gettyimages, Retrieved from
https://www.gettyimages.com/photos/coal-to-diamond
67. Figure 9-7. Antimony. Adapted from Geology Science, Retrieved from
https://geologyscience.com/minerals/antimony/
68. Figure 9-8. Mining. Adapted from The Atlantic, Retrieved from
https://www.theatlantic.com/international/archive/2015/02/the-men-who-mine-
volcanoes-indonesia/385913/
69. Figure 9-9. Uraninite.Adapted from Energy Education, Retrieved from
https://energyeducation.ca/encyclopedia/Uranium_enrichment
70. Figure 9-10. Person holding balloons. Adapted from Pinterest, Retrieved from
https://id.pinterest.com/pin/722968546417000977/
71. Figure 9-11. Cerium. Adapted from Chemistry Learner, Retrieved from
https://www.chemistrylearner.com/cerium.html
72. Figure 9-12. Plutonium. Adapted from Restricted Data, Retrieved from
http://blog.nuclearsecrecy.com/2015/04/10/critical-mass/
230