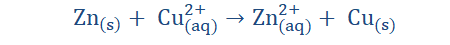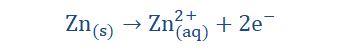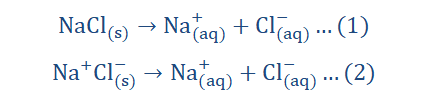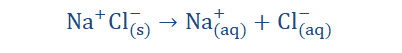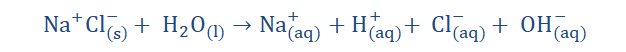CHAPTER 8: ELECTROLYSIS
If you wanted to astound me as a kid by showing me a magic trick all you had to do was tell me that you could produce electricity from water. I mean, I would not have believed you but you would certainly have blown my ten year old mind to bits with that claim. As I got older I was more and more convinced that it was all hogwash; at least until I began learning about it. You couldn’t just do it. Water and electricity could never mix…or could they?
That’s what we would be attempting to learn in this chapter. We would like to know how such ‘magic’ can come to be. However there is no magic here. We have already seen that the atom is way stranger than we expected and on that account weird things are possible. Don’t you just love chemistry?
Figure 8-1 Michael Faraday conducting an electrolytic reaction in the lab.
One of the finest minds that ever existed had its home in the skull of Michael Faraday, a scientist who made incredible advancements in the fields of electromagnetism and
electrochemistry. It is true that the concept of electrolysis had been known and
preceded his discourse on it but he had given an exhaustive description of the
phenomenon. He also coined the very term electrolysis which we now use as we please and as suggested by Rev. William Whewell, another impressive scientific mind and
polymath. Other great figures like Anthony Carlisle, William Nicholson, Alessandro 188
Volta, Humphry Davy, Luigi Galvani and a host of others contributed to the colorful history of the phenomenon known as electrolysis; which we can unfortunately not
examine here. However indulge in reading that history when you can. As your ‘invisible’
guide I assure you it is worth the effort.
So what is electrolysis all about?
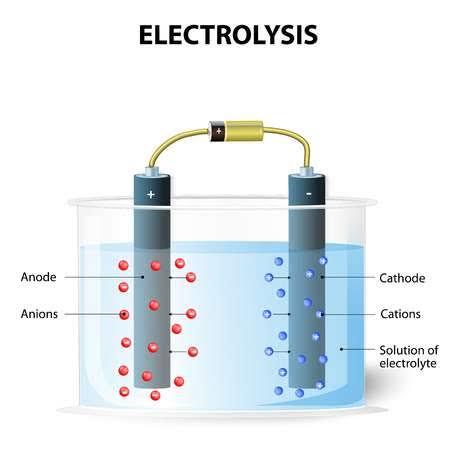
There are three important proponents to an electrolytic system. These are the
electrolyte, the direct current and external circuit and the electrode.
Figure 8-2 Electrolytic cell
The electrolyte is the substance that on dissolution produces free ions. As you know ions are charged atoms, and so they possess charges. They also carry electric currents.
“An electrolyte is a substance that produces free
ions on dissolution in water.”
Electrolytes when in solid form do not conduct electricity and so are not suitable for use as electrolytes since the ions are ‘locked’ and are not free to move about in order to conduct electricity. Direct current is passed into the solution of the electrolyte and as it does it breaks down the compound (electrolyte) into simpler substances. When this happens the ions are passed across an external circuit which consists of a battery and some wires. They do not just leap into the wires however but pass through conductors known as electrodes. There are two types of electrodes known as the anode and the
cathode (if it sounds a lot like anions and cations; it’s supposed to. You’ll soon get to know why).These electrodes allow the ions pass and then get deposited as the products of the reaction. The entire circuit is known as an electrolytic cell or circuit.
189
Now let’s put this all together and see if we can paint a vivid picture of electrolysis and reach a good definition. In electrolysis, an electric current is passed through an external circuit into a solution of an electrolyte. The electrolyte breaks down and forms free ions.
These ions pass through the appropriate electrodes through the external circuit and are deposited as the products of the electrolytic decomposition reaction (hope you
remember this).
“Electrolysis is defined as the process by which an
electrolyte undergoes decomposition when an
electric current is passed through it.”
I think that’s about right.
I am quite sure your curious mind is not satisfied with this. You want to know what’s going on at an atomic level. In that case, you are truly becoming a full blown chemist.
Walk with me.
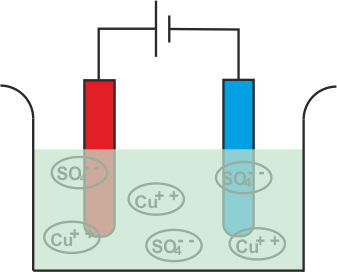
Figure 8-3 Electrolysis of CuSO4
We already know that there are three parts to this process: the electrolyte, the
electrodes and the direct current. We know their definitions and have a general idea of what they do for the process to come to be.
More specifically however, if we could just magnify the process by a large amount we would find that CuSO4 being an electrovalent compound consists of a large number of ions, some positive and others negative. The positive ions (atoms having more protons than electrons) are called cations while the negative ions (other atoms that have more 190
electrons than protons instead) are anions. In the absence of an electric current to break down the electrolyte however, neutral atoms tend to move about in the solution.
But in the event that the electrolyte breaks down we find these ions swimming aimlessly about in solution absorbed by something – the electrodes.
There are two kinds of electrodes in an electrochemical cell, just like there are two kinds of ions. There are electrodes that have an affinity (tend to attract) positive ions. Because the ions they attract are positive, it is easy to infer that these electrodes must be negative. Since they attract cat-ions they are known as cat-hodes.
On account of their similar names this singular fact confused me a lot in secondary school. I had thought that cations being positive, meant that cathodes were also positive and vice versa, but that isn’t true. Cations are positive, cathodes are negatively charged. In a similar way, an-odes attract an-ions and since anions are negatively charged, anodes are positively charged electrodes.
Sometimes a single electrode is introduced into a solution of its salt and electrode potential is set up between the solution and the electrode as in the image above (fig.3).
As seen, neutral atoms on the electrode (Copper plate in this case) lose electrons and fall into the solution as positively charged ions (cations). As more and more of them do this the electrode becomes negatively charged and the solution positively charged. As you may already know, when this happens, a potential difference is set up between them. This potential difference is what we have already referred to as the electrode potential.
Half-cell reactions: Two electrodes
It is important that you realize especially at the level you are that all you learn is not just for the purpose of passing exams or bragging about how much you know, but for the noble aim of helping to solve some problems faced by the world today using what you have acquired. Don’t be alarmed, I would only remind you of this fact throughout our discourse just so that in knowing, you will assist when and how you may now or in the not so distant future.
I have to admit though that the previous paragraph only came about because of what we would be discussing which is; electrochemical cells. The world is facing an energy crisis today. It is up to us chemists to find new ways or refine old ones so that we have sufficient energy that pose less harm to the environment. As you know energy can be transformed from one form to another. A popular energy transformation is the chemical to electrical energy conversion. The device that makes this possible is known as an 191
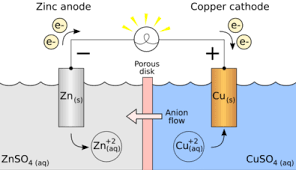
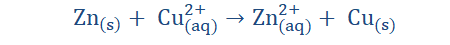
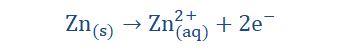
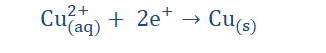
electrochemical cell. In electrolysis we could also achieve electrical energy from chemical energy. An example of such a cell is shown below.
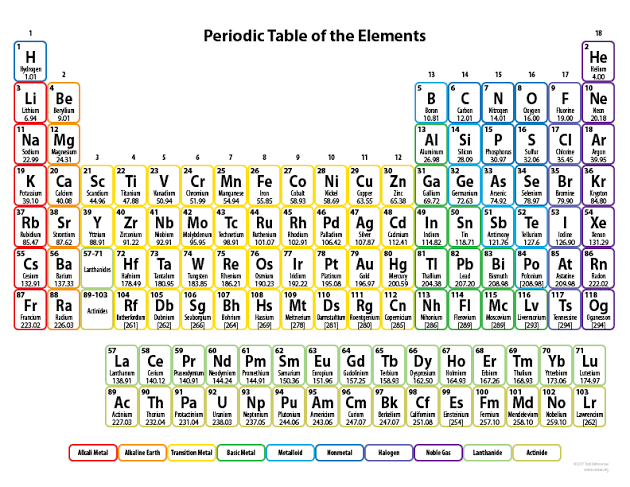
Figure 8-4 Electricity from a Zn-Cu electrochemical cell
Redox (recall reduction and oxidation reactions) take place in electrolysis because atoms either give up electrons or gain electrons to form ions in the process. In the reaction illustrated above, Zn atoms on the Zn electrode being electronegative lose two electrons each with ease and in so doing acquire a net positive charge (form cations).
These cations then go into the solution and so the solution (ZNSO4) becomes positively charged. The excess electrons however remain on the Zn electrode, making the
electrode negatively charged. From this Zn halfcell (half of the electrochemical circuit) the excess electrons on the electrode then flow along the wire to meet the Cu electrode on the other side (the second halfcell). The ions on the Cu electrode then take up the deposited electrons and become neutral atoms. Since the electrode is therefore not negatively charged, it then functions as the cathode of the reaction. We then see that the Zn electrode which is in this case the anode, becomes depleted and the cathode
‘grows’ in size for as long as the reaction occurs. Since electrons were taken up by the Zn electrode and then given away to the Cu electrode, it is safe to say that the process of oxidation (electron loss) occurs at the anode (Zn electrode) while reduction (electron gain) occurs at the cathode (Cu electrode). The complete reaction is:
The half-cell reactions are shown below. Oxidation occurs at the anode:
Reduction occurs at the cathode:
192

The Ionic theory
The behavior of electrolytes is a very interesting thing to consider when dealing with electrolysis. Why they dissociate, the form in which they conduct electricity and other factors are things we would closely look at here. So walk with me.
Figure 8-5 Svante Arrhenius
It is the year 1887 and we find the pensive Arrhenius with an experimental set up before him. He barely notices our presence. I take an interest in his set up while you read through his notes. You see that he is working on a theory, attempting to explain how electrolysis occurs by showing that the electrolyte somehow dissociates into ions when met with a direct current or melted in solution. Let’s take a look at this.
You already know that electrolysis occurs due to the passage of ions. But why is this so? Why can’t neutral atoms do this as well? Arrhenius explained this by stating that neutral atoms could not conduct electricity. The electrolyte must be dissociated into ions and a direct current must be introduced so as to align these ions, meaning that the positive ions would then be attracted to the negative electrode and likewise the negative ions would cling to the positive electrode. The dissociation that occurs in electrolytes is known as ionization which I am certain you recall.
The Arrhenius theory for a long time painted a convenient picture of electrolysis.
However it was discovered that even in solid form, strong alkalis and salts still contained oppositely charged ions, hence they did not require dissolution to be formed. It was then observed, that the freeing of the ions in solution was what was required for electrolysis 193
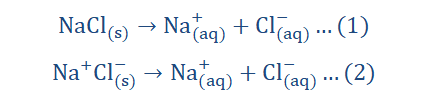
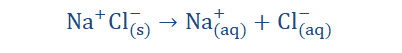
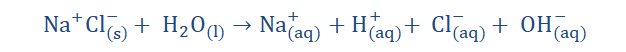
as the ions must exist in the structure even before dissolution could occur. This means that even without dissolution occurring there can exist ions.
Of the two equations listed above, which do you consider to be a more accurate
depiction of the dissolution of NaCl? I’ll wait.
“Arrhernius’ ionic theory states that when an
electrolyte is dissolved or melted in water, its
molecules dissociate into ions that move freely in
solution.”
Ions and preferential discharge
No matter how much we would like it to be so for simplicity’s sake, ions are not
discharged all willy nilly at the electrodes to form neutral atoms. Some ions are favorites and are hence preferred during ion discharge and this is due to a number of reasons which we would discuss shortly. Remember that a number of ions are usually involved in the electrolytic process as the electrolyte may contain more than one ion as in our NaCl example above where Na+ and Cl- ions are involved.
The above equation however is not complete. When expanded…
We can see from the above that there are two anions (negative ions) and two cations (positive ions) so which would be preferably discharged at each electrode? (The anode and the cathode). We need to know which ions would be the ‘chosen ones’ and why.
Below are the reasons.
194
Reasons for Preferential Discharge of Ions
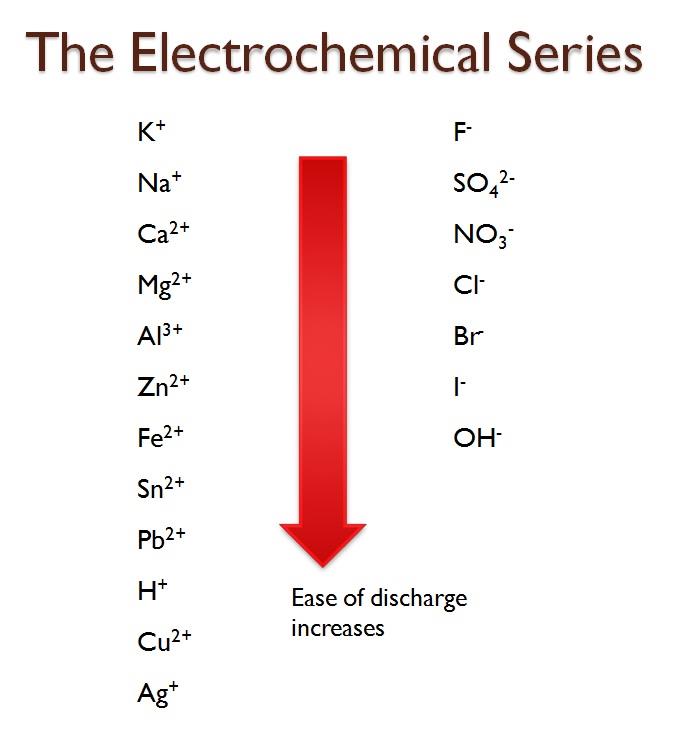
Figure 8-6 The Electrochemical Series
1. Position on the Electrochemical Series: The electrochemical series is a series that shows the arrangement of elements based on their standard electrode
potentials. For cations and anions the ease of discharge increases as you go
down the list. What this means is that cations and anions lower in the list are the easiest to be discharged when there are more than one cation or anion present
in a solution. So for instance if we have Cu2+ and Pb2+ ions in solution, Cu2+ would be preferentially discharged, leaving behind Pb ions in solution. If OH and NO -
3
are in solution, which do you think would be preferentially discharged at the
electrodes in electrolysis? I’ll wait.
2. Concentration of ions in Solution: Even in situations where one species of ions may be preferentially discharged, we find that it happening that way is not
always so. This happens because some ions are more concentrated than others
in solution and hence these ones are preferentially discharged.
3. Nature of the Electrodes: Inert electrodes are electrodes that do not partake in the electrolytic process, in the sense that they do not influence electrolytic
discharge. On the other hand electrodes like Platinum are known to be reactive
195
and hence actively influence what is being produced. This could be a determining factor in deciding or predicting ions that are deposited in the process.
Electroplating
In industrial applications, it can be quite difficult to produce and process pure metals. Things get expensive pretty fast and manufacturers have to consider
more economical methods to produce the exact (or close enough) metals and
materials with the properties they desire. For instance, a lot of ‘gold’ out there really isn’t gold at all, so sometimes your mother may put on glistening ‘golden’
earrings that are not gold, but have simply undergone electroplating.
“Electroplating is defined as the process by
which one metal is coated with or dissolved into
another by the process of electrolysis.”
With electroplating materials may be made to resist corrosion, be stronger, or just be made to be more attractive in appearance.
In electroplating the anode (positive electrode) is the metal that will be used to coat some other material. The cathode (negative electrode) is the object that
would be coated. The electrolyte is a solution containing the metal that is doing the coating. When the process of electrolysis is allowed to run, the metal object is then covered with the electroplating material and becomes a different material.
Quite delightful!
196
As usual, it is time for a refreshing beverage. I have taken to orange juices lately, they are quite healthy and great for thinking.
197
SUMMARY
Electrolysis has a colourful history, with names like Michael Faraday, Rev.
William Whewell, Anthony Carlisle, William Nicholson, Alessandro Volta,
Humphry Davy and Luigi Galvani (You can read up on it sometime).
The components to an electrolytic system are the electrolyte, the direct current, external circuit and the electrode.
An electrolyte is a substance that produces free ions on dissolution in water.
Electrolysis is defined as the process by which an electrolyte undergoes
decomposition when an electric current is passed through it.
Anodes are positively charged electrodes and cathodes are negatively charged
electrodes.
An electrochemical cell converts chemical energy to electrical energy.
Arrherius’ ionic theory states that when an electrolyte is dissolved or melted in water, its molecules dissociate into ions that move freely in solution.
Ions are preferentially discharged at the electrodes due to reasons that include position on the electrochemical series, concentration of ions in solution and the nature of the electrodes.
Electroplating is defined as the process by which one metal is coated with or
dissolved into another by the process of electrolysis.
198
MNEMONICS
Found a mnemonic today?
The reasons for preferential discharge of ions during electrolysis:
E
C
N
E– Electrochemical; as in “Position of ions on the electrochemical series. ”
C- Concentration; as in “Concentration of ions in solution. ”
N- Nature; as in “Nature of the electrodes. ”
‘Eat Curly Noodles.’ Or something else that’s not about noodles.
199
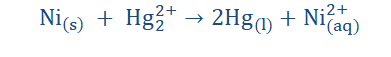
REVISION QUESTIONS
1. Define the following:
a. Electrolysis
b. Electrodes
c. Electrochemical cells
d. Electroplating
2. State and explain the Ionic theory.
3. K+ and Na+ are both present in a solution during electrolysis, which would be discharged first and why?
4. Write the half-cell reactions for the following equation:
200