CHAPTER 1: ATOMS
In all the scientific endeavors of mankind none has been quite as revolutionary as the discovery the atom. Indeed it can be argued that the most incredible ideas have
stemmed from this field. We are about to dive into a miniature world of particles so small that on the very tip of a needle can be found millions and millions of them. They make up all that we see and despite this appear virtually invisible and can only be observed using very controlled type of experiments. The crazy thing about atoms is that this isn’t even the most spectacular feature they possess. We will now delve into their colorful history and note some of these exciting features we talked about. Exams may be
tedious but you will be swept away by the world you are about to enter so much that you will be sure to emerge an ecstatic scientist yourself.
A BRIEF HISTORY OF THE ATOM (OR THE MADNESS OF THE ATOM)
One could say the macro world is easily observable and so it is no difficult challenge to observe the things that take place around us except in reality…that would be erroneous.
Despite the fact that we can clearly see the things around us we have made several errors when coming to an understanding of these things because they can be difficult to explain. For instance in order to explain lightening the ancient Greeks had adopted the 6

belief that a superhuman in the heavens threw bolts at the earth, their god they knew as Zeus. The ancient Mesopotamians believed that the sky was held up by pillars as in a temple. Let’s fast forward to relatively recent times. It was once believed in the 1800’s that lead was a wonder substance and so people used it to make hats, coat plates and even swallow it whole to attain beauty! In relation to the lead craze there is a story about people in England who kept dying from consuming tomatoes. Witches were said to use tomatoes to cause those deaths. It was only realized later that the poor tomatoes were not the culprits, the plates were as they were generously coated with lead. So one can see that even the visible world that we can see and touch can be difficult to examine in its entirety. How then could we possibly study micro particles invisible to the eye? And what’s more how did we even come to know about them?
Figure 1-1 "Zeus must be really angry guys!" A Greek man probably once said.
The Greeks who could see the invisible
In many texts on the atom the Greeks are seldom mentioned and it was they who
began to observe that something was not quite so obvious in the nature of things. In fact the very word ‘atom’ finds its origin in Greek text and Greek language as
‘atomos’ meaning indivisible. So we see that the ancients were certainly keen on an idea here. Up until a few hundred years later the atom was indeed considered
indivisible and composing all matter and we must give them credit because figuring that out in as early as 460 BC is extraordinary.
7

Two of these wise ancients are specifically credited with the discovery of the atom.
However, that is only based on the data we have. These two are Leucippus and
Democritus, the latter a student of the former. They also added that atoms were of different kinds depending on what properties the matter they made had. For instance the atom of water was considered wet and slippery and iron atoms were metallic
with hooks. So we can imagine that the atoms of fire could burn. While this may
sound ridiculous to modern day scientists as ourselves we must realize how ancient this was. It was way before Jesus had been born! Nonetheless these theories set
more modern scientists on a trail and I emphasize the word ‘modern’ because from
the time the Greeks noted this to more recent times there was little improvement by way of study on the subject but in 1803 that was to change.
John Dalton, the new Leucippus but not exactly
Figure 1-2 John Dalton in a stylish pose
8
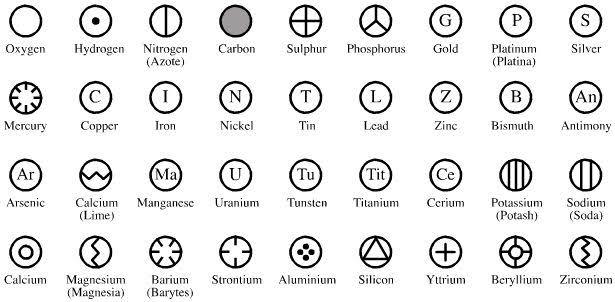
Figure 1-3 John Dalton’s symbols for the known elements at the time
A chemist and school teacher who had read the works of the ancient Greeks (most
likely) had improved on the works of Leucippus and Democritus stating that atoms
were indivisible, small hard spheres and the atoms of particular elements were identical to each other. You should hold on to that definition as several points would be ironed out later. He also documented a list of the first chemical symbols of the few discovered elements. He even stated the very first postulates of the atomic theory:
1. Matter consists of indivisible particles known as atoms.
2. Atoms of the same element show similarities in all ways but differ from atoms of other elements.
3. Atoms can neither be created nor destroyed.
4. When atoms combine, they do so in simple whole number ratios to form
compounds.
5. The atom is the smallest unit of matter that can participate in a chemical
reaction.
So there we have it. The above postulates are the very basis on which modern
chemistry is built and we are only just getting started. Before we move on it would help if you stick to this definition for the time being:
“The atom is the smallest unit of matter that can
participate in a chemical reaction.”
You’d thank me later.
9
The boom of the 1900’s
Okay technically it began in the late 1800’s. We are not entirely certain what John Dalton did in addition to the discoveries he made but he seemed to do something extra like maybe set off an investigation bomb because almost right after his study everyone wanted to gain in on the atom action. It was discovery after discovery and I am sure that if social media existed our phones would have been ‘blown’ from the overwhelming
wealth of new discoveries. Walk with me.
Beginning at the end of the 1800’s in the year 1897, we see a studiously engaged
Joseph John (J.J) Thompson bent over a cathode ray tube and inspecting it. He has just made a tremendous discovery. He has discovered that unlike what our ancient Greeks and John Dalton believed the atom isn’t indivisible. It can be divided and on this
‘division’ we notice a negatively charged particle of significant mass which he called a
‘corpuscle’ and we are glad that name did not catch on. However it was a most
significant breakthrough. Below is an image showing how this experiment was
conducted and don’t worry we will walk through the process carefully and I’ll have to explain because I am sure Thompson won’t have the time (He’s second guessing the
name).
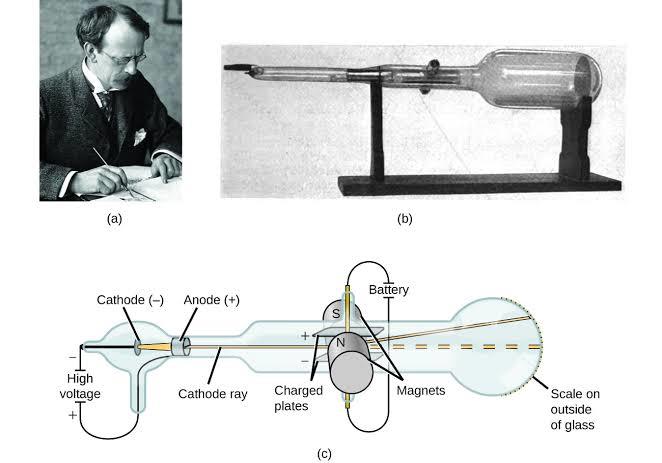
Figure 1-4(a) J.J Thomson, (b) the actual cathode ray tube used, (c) A schematic showing how it works.
Before diving into the discovery of the electron, let’s come to understand and appreciate the cathode ray tube. As seen in the image above a cathode ray tube is well, a tube. It is a tube made of glass and with wires connected at its ends. Within the glass
10
compartment is a vacuum (meaning all the air is pumped out) and an electric charge passes through the tube forming a glow.
Before Thompson’s experiment many people had known about the cathode ray tube
and the glow (rays) produced. Thompson however wondered if this ray was all there was to notice. He then decided to customize a cathode ray tube, adding charged plates on either end. The plates were then given two slits that connected to a scale outside the glass which could be used to measure charge as shown above. On applying a
magnetic field across his fine tube he noticed something incredible. The rays had deviated from the straight path. What this showed was that the charge had been
affected by the magnetic field.
“So the ray and the charge are as intertwined as pizza and cheese.” He said probably, stroking his chin. (He didn’t.)
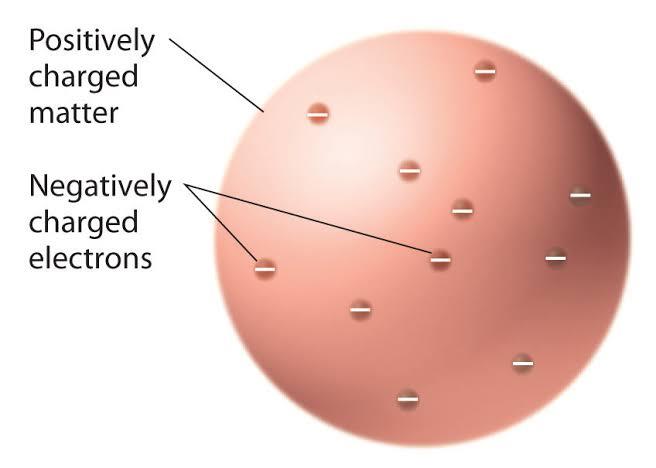
What he had just discovered was that the ‘rays’ were actually particles as they could be affected by a magnetic field. Further experiments showed that these ‘corpuscles’ which we would rather call ‘electrons’ were relatively light(lighter than hydrogen, the lightest element) as well as negatively charged.He later put forward a model of what he
conceived the atom to be, and no it had no metal hooks as the ancient Greeks thought instead it was a positively charged sphere with electrons embedded.
Figure 1-5 The J.J Thomson model of the atom.
This won him the 1906 Nobel Prize which he rightly deserved. So we have met the
electron and we should hold on to an important property of it.
11

“Electrons are negatively charged particles.”
Rutherford Shoots Gold
I still believe this was quite an expensive experiment and you may just agree. Just like Leucippus and Democritus, Ernest Rutherford worked and studied under J.J Thomson.
His mentor and teacher had probed into just the surface of the atom and he caught some of that atom fever. In the 1911 experiment which he performed with two of his colleagues Geiger and Marsden, he fired alpha particles at thin sheets of gold foil. Now, alpha particles would be discussed later under radioactivity but what you should know now is that these particles are positive and positive charges are repelled by other positive charges but attracted to negative charges. In addition, alpha particles are quite heavy so it’ll take a lot to deflect them off their course. So walk with me.
Figure 1-6 Ernest Rutherford
Now, if the atom is a sphere of positive charge as Thomson observed, we should expect that the fired alpha particles would pass through the gold foil sheet (alpha particles are so tiny, they could) and show little to no deflection but this well, was not the case and we can see the surprise on Rutherford’s face. Can you see it? I see it every time I mentally walk by him.
Turns out that most of the alpha particles fired passed through the gold foil. This was not something to make him widen his gaze at the screen, but then the unexpected
happened. A few of those particles became deflected but not just deflected, deflected at alarming angles as though the footballer Messi had kicked them hard. We can now walk over to the desk of the great man and bend over his shoulders. In his notes we can see that he is describing the experiment; “It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you.” Extraordinary. It is 12
certainly not something anyone should try but I can’t imagine shooting a tissue paper with a gun and expecting the bullet to come back and hit me but then I suppose it is probably more economical than firing gold. Rutherford’s interpretation of this experiment was that unlike what his mentor had postulated, the atom was not all positively
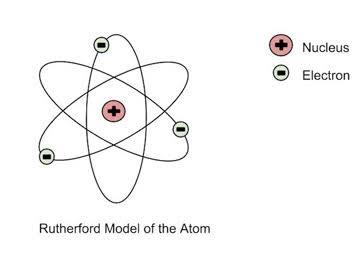
dispersed charged but the charge was concentrated in the middle, a small very dense centre known as the nucleus and the atom itself is mostly composed of empty space.
This was such an astounding discovery especially as it happened in only a few years since Thomson’s incredible studies. Rutherford naturally had to make some changes to the existing idea of what the atom looked like based on Thomson’s work.
Figure 1-7 Rutherford’s atom.
He proposed that the electrons orbited the positively charged nucleus that was situated at the center of the atom.
“The nucleus is the positively charged center of the
atom, composed of protons.”
Niels Bohr on another level
Thomson and Rutherford were great scientists and everyone knew that but despite how significant their studies were something was just not quite right. According to
fundamental scientific laws, spiraling electrons should not remain spiraling around the nucleus and should crash pretty soon like a jet engine in the air out of fuel but Rutherford’s atomic model could not explain this. It wasn’t magic that kept electrons 13
moving about and someone had to find out what. Then in came Niels Bohr and now it’s time to walk with me.
We enter a room and find the man facing a board. We see strange drawings just like the ones below.
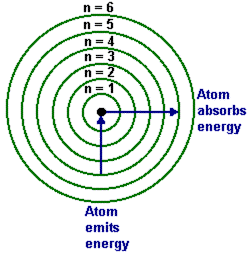
Figure 1-8 Atomic energy levels.
He seems to be frantically writing so I’ll explain what is going on. You have probably heard about quantum theory. No? Well it is another of those insane little concepts that have been conceived because the atom is just so strange. It was brought about by a man named Max Plank, who observed in an experiment that radiation was carried in
‘packets’ called quanta. Don’t leave just yet, stay with me. We would explain quanta in a while but for now just picture indomie packets. Each packet can only hold so much indomie and not more. Energy according to Max plank was not an indefinite stream as was believed by most but ‘indomie packets’ of energy instead.
The work of Niels Bohr was based on this concept. He noted that the reason the
electrons did not ‘crash and burn’ into the nucleus was that the electrons could only be located on particular energy levels. So the energy of electrons was quantized or could be said to fit into particular ‘electron indomie packets’ -which sounds like a good name for a new indomie package. Electrons couldn’t just assume any random value but had to remain at these levels and could only move between them. If an electron had acquired energy (maybe due to heating) it could ‘jump’ to a higher energy level but on releasing that energy it would fall back to its energy state. The ground state or ground level was defined as the lowest energy level an electron could go as you can see in the image above.
At this point in this amazing history we could go on to a happily ever after moment and say that everything was fine and that Bohr was the hero of the day, the last avenger who stood up to Thanos and defeated him (Get the reference?) but unfortunately that is seldom the case in the story of the atom. When scientists thought they cracked a code, 14
they only opened it to find more puzzling problems. The atom has well become a miniature Pandora ’s Box.
Niels Bohr’s model worked amazingly when applied to the hydrogen atom, but when
scientists began involving heavier elements they found more and more complications.
We would explain these complications a little later. For now, we should remember that we cannot underestimate the work of Niels Bohr because it helps us understand
chemistry at least at the fundamental level. A thorough grasp of Niels Bohr’s atomic model is a grasp of chemical bonding and reactivity rates of elements in the periodic table. Since the aim of this text is to narrate the history leading to our present knowledge of the atom, walk with me as we strive to get to the Standard Model of the Atom.
Schrodinger traps a cat
Of course the atomic Pandora’s Box had only just begun.After Niels Bohr had taken a crack at this atom wonder, another scientist by the name of Erwin Schrodinger became infected with the fever and set to work trying to straighten out the rumples of Bohr’s atom. In 1926 he proposed that electrons instead of being imagined as particles should be pictured as waves instead. He used a plethora of equations to explain this and ended up with a picture of the electrons not as distinct particles but as electron clouds.
As much as we would love to go into his study and watch him work on coming up with these equations, I’d rather not and am sure you won’t want to either, at least not just yet. Chemistry unfolds in layers and soon even Schrodinger’s equations would be
nothing to fret over.
15

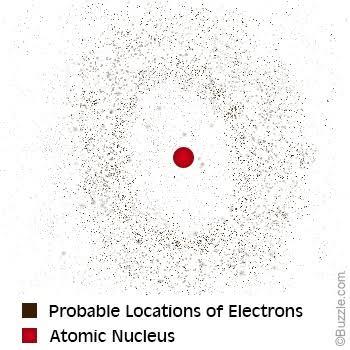
Figure 1-9Erwin Schrodinger
Figure 1-10 The electron cloud
Nonetheless Schrodinger showed that the clouds were only just estimates of the
electron’s position because we can’t really tell where the electrons are. We can only guess likely areas where we may find them. I think this makes sense because you can imagine losing a small marble in a large cotton candy cloud of identical small marbles.
Good luck finding it. These areas where electrons may likely be found have been called electron orbitals. You certainly would not want to forget this.
16
“Electron orbitals are regions within an atom where
an electron would most likely be found.”
We’d talk about this really soon.
Now I know what you’re thinking. What does all this have to do with a cat and a box?
Well, just like Schrodinger’s equations, it is safe to say that that is well beyond our scope at least for now but remain curious and you’d find out.
James Chadwick, the other Democritus
So after J.J Thomson had discovered the electron and proposed his model of the atom and Ernest Rutherford, a student of his discovered the nucleus and proposed his own model of the atom, the ‘atom breaking boys’ were far from done as a third person in the line, a student of Ernest Rutherford discovered an important part of the atom, one that would have made John Dalton take back some points in his postulates if he had known about it. The student was James Chadwick and that discovery is the neutron. It has no charge (neutral) and shares the nucleus with the proton, while the electron surrounds it.
“The neutron has no charge.”
With the discovery of the nucleus came the knowledge that unlike John Dalton’s
postulate, atoms of an element could differ based on the number of neutrons they possess. This is called Isotopy. Before you understand isotopy however, you must come to terms with the concepts of atomic and mass numbers.
“Atomic number is the number of protons in the
nucleus of an atom.”
The atomic number was observed to be the reason for the characteristics an element possessed.
17
“Mass number is the number of protons and
neutrons in the nucleus of an atom.”
And now…
“Isotopy is the existence of atoms that have the
same atomic numbers but different molecular
masses (mass numbers) due to a difference in the
number of neutrons.”
And now, we could paint a complete picture of the nature of the atom, but not just yet.
The Standard Model
If you thought the atom was crazy, think again as crazy is an understatement. Most secondary level textbooks don’t venture into this area but we are hardened scientists and we would cut through the thick jungle until we arrive at our treasure or at least have a glimpse of it, which is the aim of this section.
The standard Model came into the scene in as recent as the 1970’s. After all the
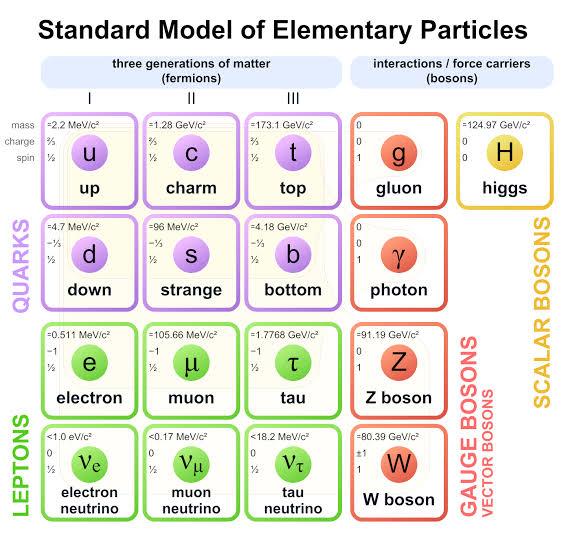
craziness of the atom scientists continued to dig deeper and deeper but no matter how much digging was done they continued to meet hard ground. Nonetheless the effort was seldom futile as more discoveries opened up the atom’s mysteries. The chart below shows the particles of the standard model.
Figure 1-11Standard Model chart.
According to this model a number of particles explain how the world and the universe work. These are fermions, bosons, quarks, leptons, anti-quarks…. It may seem like a lot 18
to take in. Why not drop down this book for a quick, refreshing cold drink first and come back in a bit so that we walk through the standard model, at least briefly?
Back to briefly exploring the Standard Model
Hope you are hydrated and well rested now because you are going to need quite the effort to grasp some of the final madness of the atom. Don’t worry it isn’t hard, you’ve just got to exercise some imagination.
We seemed to be speaking a strange language a while back. I mean electrons, protons and neutrons we could manage but fermions? Bosons? Queen Elizabeth? Now that’s
too much but remember that all the high sounding words are actually easy when you get to know them. So don’t fret, but enjoy what you learn.
“Knowledge which is acquired under compulsion,
obtains no hold on the mind.” –Plato
With Plato’s guiding words, let’s begin.
The standard model is the most recent advance towards deducing the nature of atoms.
In time the atom fever became even more intense. A lot of money was pumped into
research in order to understand whatever was going on because it seemed that the
atom would never be short of mysteries. The atom went from being the simple model to one that required quantum mechanics to understand. Several questions went
unanswered. One of these was, “What holds the protons together in the nucleus when they should pull apart having the same charge?” Even the neutron could not completely account for this. As time passed several other particles were observed in a plethora of 19
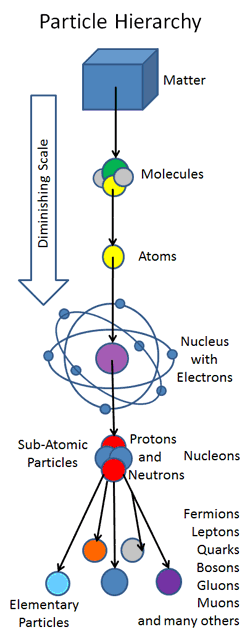
experiments. The photon was then added to these fundamental particles, described by the famous Albert Einstein, followed by the positron observed by Anderson then the pion (holds the nucleus together, answering our initial question) by Yukawa, the muon by I.I Rabi and the list went on until hundreds of these basic particles were discovered.
I can only imagine what schooling would be like if you were asked to list these particles.
Quite sure I just may have dropped out because that won’t be funny.
After decades and decades of work the overwhelming number of these fundamental
particles became more concise, easy to manage and kept me in school. These particles make up the Standard Model as you have seen in the table above. They are only a few and their interactions and compositions are summarized in the image below.
Figure 1-12 Relation between the Standard Model particles.
This concludes a brief history of the atom and yes it is brief because on account of our scope quite some information was left out. Don’t stop being curious however and don’t get tired because we’ve got quite a long, interesting way ahead of us.
20
SUMMARY:
The ancient Greeks were the first to juggle with the concept of an atom. They
called it ‘atomos’ meaning indivisible. Leucippus and his student Democritus are
credited with the discovery.
In 1803 John Dalton published the first postulates of the atomic theory and
proposed symbols for the known chemical elements at the time.
Joseph John Thomson discovered the electron in his cathode ray tube
experiment, he observed that they are negatively charged and proposed the
structure of the atom to be a positively charged sphere with electrons embedded
like a pie with resins.
Ernest Rutherford who was a student of J.J Thomson discovered the nucleus of
the atom in an experiment and learnt that the atom is mostly empty space. His
model of the atom was a dense, central nucleus with electrons spiraling around
it.
Harnessing Max Plank’s concept of quanta, Niels Bohr proposed that the electrons occupy specific energy levels.
Erwin Schrodinger suggested the existence of energy clouds as opposed to particulate electrons and explained electron orbitals. Electrons are therefore more wavelike. He also did talk about a cat, but it is beyond our scope.
James Chadwick, a student of Ernest Rutherford discoveredthe neutron. It has
no charge (is neutral) and resides in the nucleus with the proton. With the
discovery of the neutron isotopy came to be and so did the concepts atomic and mass numbers.
The standard model is a lot…of fun.
The fermions, bosons and a host of particles make up the standard model.
Forces are also accounted for by this model of the atom.
21
MNEMONICS
Learning stuff can be hard especially when there’s so much of it. That’s why at the end of every chapter you’ll find a few suggestions to aid memorizing lists and processes so that while you learn and have fun you also retain information that would be useful for exams.
John Dalton’s postulates of the atomic theory:
A
S
N
R
S
A – Atoms: As in “Matter is composed of Atoms.”
S – Similarities: As in “Atoms of the same element show Similarities in all ways but differ from atoms of other elements.”
N – Neither: As in “Can Neither be created nor destroyed.”
S – Smallest: As in “The atom is the Smallest unit of matter that can participate in a chemical reaction.”
And now there are numerous possibilities for what you choose to be a mnemonic. It could be “All Snowboards Never Raid Stores”. Ridiculous right? And now all I can think about is snowboards on two feet with ski masks not robbing some unfortunate Hawaiian man by the beach selling well…snowboards but those same snowboard people robbing
banks. But you may like to connect the mnemonic with the subject so it could become;
“Atomic Snowboards Never Raid Stores.”In this case it is connected to our subject and now I can picture John Dalton, the leader of a gang of snowboards with spiraling
spheres about them robbing banks. A third option is, “Atoms Show New Research
Sometimes.” This approach is more direct and no nonsense. However I would suggest some crazy mnemonics. The crazier they are, the more likely they are to stick to mind.
22
REVISION QUESTIONS
1. Define an atom according to John Dalton.
2. What were previously thought to be the three fundamental particles of the atom?
3. State the names of the scientists who discovered the following:
The electron
The proton
The nucleus
The positron
The neutron
The muon
The pion
4. Who discovered the atom?
5. What are the postulates of the atomic theory as stated by John Dalton?
6. Describe in a few words the structure of the atom according to the following scientists:
Erwin Schrodinger
J.J Thomson
Ernest Rutherford
Niels Bohr
7. What is an electron orbital?
8. Define the atomic and mass numbers of an element.
9. What is isotopy?
10. Based on this brief introduction, what do you suppose the future holds for the atomic theory? Do you believe the standard model is a final stop on the road or
do you think there is more of the madness in store? Give reasons for your
answer and don’t forget to be imaginative.
23