CHAPTER 2: MORE ON ATOMS
Reactions, Moles and Equations
Glad to see you’ve returned. I am quite sure you must be scratching your head after seeing the topic of this chapter but you needn’t fret. The atom is a broad subject indeed but that makes sense right? I mean the atom makes up all matter so it is no wonder that it is so wide in scope.
In the last chapter we very briefly discussed the history of the atom. I say briefly because there is still so much to know if you want to. In this chapter we would be talking about how to represent these atoms and understand them both qualitatively and
quantitatively. First off now, we would define some important terms that you would need to understand all we’re going to do. Get ready; we would be speaking in the chemistry language shortly.
“An atom is the smallest unit of a substance that can
participate in a chemical reaction.”
Remember that? I sure hope you do because you’d need it plenty.
“A molecule is a group composed of two or more
chemically bonded atoms.”
24
What this means is that when two or more atoms come together they form molecules.
The atoms that form a molecule may be of the same or different kinds. For instance if I invited a friend over for arts and crafts and somehow for reasons unknown our jackets became glued together, I alone may just be considered an ‘atom’ but the both of us together chemically bonded (Would explain this later) could be labeled a ‘molecule’. If I played around with a single football and got bored quickly and then in a spark of ‘genius’
decided to glue the football and a basketball together, the single ball could be called an
‘atom’ while the two balls together ( in chemical bond) would be a molecule. If I decide that even two balls have little fun potential and add another, maybe a baseball, there would be three separate atoms that chemically bonded together would become a
molecule. Now I hope you realize that people and balls do not constitute atoms, but this is just to give you an idea of what atoms and molecules are like. Do not forget the words
‘chemical bonds’ because in time you will realize that things can be combined in more than one way, and a chemical bond is just one of them.
“Elements are pure chemical substances that cannot
be broken down into simpler substances by ordinary
chemical means.”
Okay, that’s a mouthful. Let’s break it down.
Atoms when chemically combined make up molecules. Atoms of the same kind when held together make up elements and these elements cannot be broken down by any old chemical methods because they are pure chemical substances. Hmm… Something’s
missing here but can’t put my finger on it…
“Compounds are substances that are formed when
two or more elements are chemically bonded.”
And lastly;
“A solution is a homogenous mixture of substances
(solutes) in which the constituents (atoms and
25
molecules) of the substance are dispersed
completely in a solvent.”
That’s it! And now we have a complete picture.
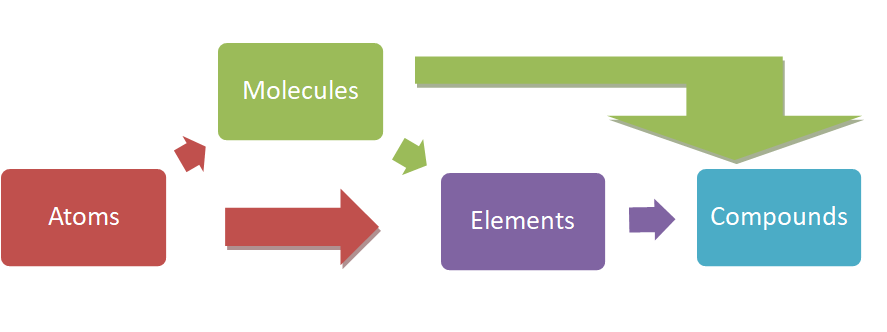
Atoms make up molecules when they are chemically bonded together. Atoms of the
same kind make up elements and elements make up compounds via chemical bonds.
Solutions contain solutes which are composed of atoms and molecules that are uniformly dispersed in a solvent. Neat right?
Figure 2-1 (In Shakespeare’s voice) “Atoms beget molecules, solutions and elements and molecules also beget solutions, elements and compounds and elements beget
compounds.”
Now that we have known what they are, how about finding some examples? Clearly
human beings and balls aren’t atoms even though they are made of them. We’ve been talking about them and I’ve seldom given an example but that’s because it’s easy to get carried away by names and not know exactly what you’re talking about.
What are Atoms and Elements really?
Usually we begin our discussions with the atom, but this time we would be giving
instances of the elements and it is only in that way that we would come to understand the atom.
The table below is the Periodic Table.
26
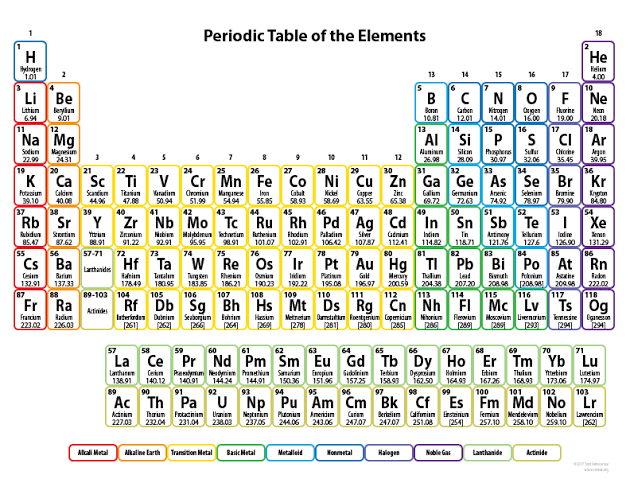
Figure 2-2 The Periodic Table
It is a table that shows all known elements discovered as well as their atomic and mass numbers. It would be covered in detail later but for now our focus lies in
understanding what those little boxes with the letters in them are. Each little box houses an element. The elements are shown by those little letters. The H in the top most left up there is the element hydrogen while the little red box below it labeled Li is lithium and so on. Elements are all around us so don’t let this weird little diagram confuse you. For instance if you look towards the upper right, in the third box to the right located at the second row, you find an O. Know what that O stands for? It stands for Oxygen. And I’m sure you probably already know that oxygen is the stuff that keeps us alive, the gas we breathe in through our noses and into our lungs and it is all around us. It has to be, else we may all be dead. So now as a mini assignment can you name at least ten elements? It’s okay to look at the table
because you only just understand the concept.
How would this help you understand an atom? We’re getting there.
Atoms are the smallest unit of a substance that can take part in a chemical reaction.
Elements can take part in chemical reactions. The smallest unit of an element can be represented by a single letter such as O for oxygen therefore an atom of oxygen is ‘O’. Isn’t that simple enough? What then is an atom of carbon? If you don’t know what carbon is just look up at the table. I’ll wait.
27
I’m no psychic but I can just tell you got that right. Now if you are asked what the symbol of an atom of carbon looks like you can just write down ‘C’ and look like a boss while showing off your John Dalton pose (refer to fig. 2, Chapter 1). Could you also say what the symbols for the atoms hydrogen and oxygen are too?
One plus one equals Molecules
That isn’t exactly true but it’s just a reminder that chemistry isn’t difficult when you understand. In fact it is as easy as 1 + 1. Now that we know what atoms are, what are molecules? What would a molecule of oxygen look like for instance? Let’s take a look at that.
O - Atom
O + O → O2 (Molecule) …(1)
And magic! Just like a simple 1 + 1, your single oxygen atoms have become a
molecule of oxygen, meaning 2 atoms. This is getting exciting. What else can we
play with? Let’s try hydrogen.
H – Atom
H + H → H2 (Molecule) …(2)
Just wonderful. I am feeling a little ‘mad sciency’. Why don’t we go all Frankenstein and combine them? (The atoms I mean).
H + O → H2O (Molecule)…(3)
Believe it or not, that little H2O right there is a molecule of water but don’t bother looking for it your bathtub because you won’t find it. On the tip of a needle alone there are millions of molecules so imagine how many molecules could fill your
bathtub…or don’t imagine so you don’t get a headache.
As much as we feel some mad science vibes here, we can’t go about doing
whatever we like. Nature is governed by rules that only the Creator can break and so we can’t combine atoms and elements all willy-nilly. The rules of combination will soon be dealt with and you’ll see why.
No compound troubles with Compounds
We already know that when elements are chemically bonded they make
compounds. You have even come across one of these compounds even though you
may not realize it. Remember when we said that only atoms of the same kind can form elements? We emphasized the words ‘same kind’ for a reason. You see, when
elements come together they form compounds but these elements must be of
different kinds (meaning they must also possess atoms of different kinds) and when 28
they do, they form compounds. An example of these compounds is water or as you now know, H2O. Can you tell why water is a compound? I’ll wait.
Water is a compound because it contains two elements: Hydrogen (H) and oxygen
(O). Another example of a compound is carbondioxide(the air we breathe out of our lungs contains this) formed from the chemical combination of elements carbon (C)
and oxygen (O).
C – Atom
O – Atom
C + O → CO2 (compound molecule).…(4)
Just like an element, a compound can be a molecule.
You may have noticed the numeral subscripts beside some of the elements, like
H2O and CO2. This shows the fixed ratio of the combination as a result of their chemical bonds. We would revisit that in another section.
Chemical Equations
Congratulations! Don’t look so surprised. You move quickly! Before coming to this section you’ve already known what a chemical equation is. I’ll just refresh your
memory.
“A chemical equation is a written representation of
a chemical reaction.”
If you understood that, you would realize that all the equations we have written are chemical equations. They show you what and what form what and are extensively used by chemists every day. Chemical equations represent chemical reactions. So
what are chemical reactions?
“A chemical reaction is a process that involves the
combination of one or more substances to yield
one or more resultant substances.”
What this implies is that when the oxygen atoms combined, they yielded the oxygen molecule in a chemical reaction and when carbon and oxygen combined they were
converted into carbondioxide in a chemical reaction.
29

With all this talk of elements and compounds, it is easy to get the two mixed up. We certainly do not want that because that could get us very confused. In the table
below, the differences between elements and compounds are broken down.
Refer to it whenever you need to.
Element
Compound
1. An element is made of only one
Compounds are composed of different
type of atom.
atoms of different elements in chemical
combination.
2. Elements cannot be broken down
Compounds can be broken down into
using chemical means or methods.
simpler compositions or substances.
3. There are only a fixed number of
Compounds are almost limitless, as
known elements in existence, only
several chemical combinations of
about 117.
elements are possible.
4. Elements are represented using
Chemical formulas represent compounds
symbols such as ‘O’.
as formulas are a combination of the
symbols (the elements), such as CO2.
Table 2.1 Differences between elements and compounds
Achieve balance with chemical equations
Now that we know what chemical equations are and can differentiate between
elements and compounds, the next crucial step is learning how to balance a
chemical equation. Just like a children’s see-saw chemical equations can be
balanced and should be or else something (or someone) may fall off.
Figure 2-3This is what an unbalanced chemical equation appears like.
Let’s revisit equation (3) showing the formation of a water molecule one more time.
30
H + O → H2O
Look carefully at that equation? What can you observe? I’ll wait.
Indeed. Something is definitely wrong. Here we can find one hydrogen atom and one oxygen atom on the left side of the equation (before the arrow) but on the right side after the arrow we see one oxygen atom and then two hydrogen atoms! That isn’t right. It’s like saying 1 + 1 = 3; just doesn’t happen. We’ve got to make this right; we’ve got to balance the equation.
2H2 + O2 → 2H2O…(5)
What do you see now? We have added another hydrogen to the one before the
arrow on the left hand side of the equation (The little ‘2’ subscript) added a ‘2’ before the hydrogen molecule. That ‘bigger 2’ represents a quantity of measurement
known as the mole and the H2 shows that there are two atoms of hydrogen, corresponding to one mole of hydrogen. The ‘2’ before hydrogen shows that there
are 2 of the H2 molecule present, meaning 2 moles.
You must be wondering about the subscripts that seemed to appear out of nowhere.
Those are there because hydrogen and oxygen are diatomic. What this means is that in their most stable form oxygen and hydrogen each exist as 2 atoms making a molecule. They cannot exist as single atoms for very long because those are
unstable. All the other elements of the periodic table have their own stable states.
Some are triatomic (show stability with a molecule of three atoms) and others are even monoatomic (single atom). This would be discussed later at length.
H – Atom
H2 – Molecule (diatomic)
H2 – One mole of hydrogen
2H2 – Two moles of hydrogen
2H2 – Four atoms of hydrogen (i.e. first 2 × second 2), two moles of hydrogen
In the final equation, we now find four hydrogen atoms and two oxygen atoms while on the right hand side we find the same number of elements now combined into the
product water( the big ‘2’ in front of hydrogen also means ‘2’ for oxygen). The
equation can now be said to be balanced. One final thing is missing though, and that is the absence of the states of the elements. What do you think this means? It means that we’ve got to know if the elements represented are solid, liquid or gas.
2H2 (g) + O2(g) → 2H2O (l)
31
The little (g) represents the term ‘gas’ showing that hydrogen and oxygen are both in the gaseous states in this reaction while (l) represents liquid, showing that the water formed shown on the right had side of the equation is in the liquid state.
It takes a lot of effort talking about ‘right sides’ and ‘left sides’ of the equation. I think we should make it easier on ourselves by calling them reactants and products of the reaction.
“Reactants are substances that undergo a
chemical reaction to give products. They are
shown on the left side of the equation.”
While…
“Products are formed when reactants undergo a
chemical reaction. They are found on the right side
of the equation.”
So in the above example, hydrogen and oxygen are the reactants, while water is the product formed. Now that we know what reactants and products are, the definition of a chemical reaction would change only so slightly;
A chemical reaction is a process that involves the
conversion of one or more reactants to yield one
or more products.”
Now why not return to the equation for the formation of CO2 and try to fix it?
Avogadro and his moles (Not the animal kind)
Have a quick glance at the equation below:
2H2 (g) + 2O2 (g) →2H2O2 (l) …(7)
What smells fishy? It isn’t moles; I don’t think they smell like fish. You have seen these bigger numbers standing before the elements before. While we explained how
32
they balanced the equation, we did not explain what they were in detail. These numbers are moles. However to truly understand the mole we must go first to the work space of an impressive scientist named Amedeo Avogadro. Walk with me.
Amadeo Avogadro was an Italian scientist who while studying gases formulated the
law known as Avogadro’s law. This law states that gases of equal volume at the
same temperature and pressure contain the same number of molecules. It is an
essential law in chemistry and has given rise to a multitude of important findings.
While he busies himself with measuring gas temperature, pressure and volume, let’s revisit the statement of this law and keep it in mind.
“Avogadro’s law states that equal volumes of all
gases at the same temperature and pressure
contain the same number of entities or units.”
This number known as Avogadro’s number is 6.02214076 × 1023 and the entities or units mentioned could be atoms, molecules, electrons or ions. (You would soon come to know what ions are, so don’t worry).
And this now leads to the definition of a mole.
“A mole is defined as the mass of a substance that
contains 6.02 × 1023 units (molecules, atoms,
electrons or ions) of that substance.”
This definition looks pretty on paper but it can be very difficult to work with
experimentally speaking. While I can say that the same volume of oxygen and
hydrogen have the same number of entities, it would be difficult to find a central value for this measurement as the atomic weight of oxygen differs from that of
hydrogen. Scientists therefore agreed on a standard, and that standard is 12 grams of the carbon-12 isotope.
Don’t fret, I’ll explain. You may remember that we defined isotopy in chapter one. I’ll juggle your memory.
33
“Isotopy is the existence of atoms that have the
same atomic numbers but different molecular
masses (mass numbers) due to a difference in the
number of neutrons.”
Carbon has three isotopes: carbon-12, carbon-13 and carbon-14. The numbers 12, 13
and 14 are the mass numbers of the elements. If you remember correctly…
“Mass number is the number of protons and
neutrons in the nucleus of an atom.”
The reason why the mass numbers differ is that they all have different neutron numbers.
The atomic number for carbon is 6. Carbon-12 therefore has 6 neutrons and carbon-13
has 7 neutrons. How many neutrons does the carbon-14 isotope possess? Remember
that:
Mass number = Number of protons + Number of neutrons
12 grams of the carbon-12 isotope has been chosen as the mole standard, altering our definition of the mole slightly to:
“The mole is the amount of a substance that
contains as many elementary particles or units as
they are in 12 grams of the carbon-12 isotope.”
If this seems too complicated to get your head around, an easier way to remember the mole is to know that the mass number (or atomic mass) in grams of an element is
equivalent to 1 mole of that element. For instance;
Atomic mass of oxygen – 16
Atomic mass of oxygen in grams – 16 grams
1 mole of oxygen (O2 ) = 16 grams
2 moles of oxygen (2O2 ) = 32 grams (16 grams × 2)
34
We therefore see that 16 grams (1 mole) of oxygen contains as many elementary particles as they are in 12 grams of the Carbon-12 isotope.
In the case of compounds such as H2O;
Atomic mass of hydrogen (H) = 1
Atomic mass of oxygen (O) = 16
Relative molecular mass of H2O – 1(atomic number of H) × 2(number of H atoms) + 16
(atomic mass of O) = 18 grams
1 mole of water (H2O) = 18 grams
2 moles of water (2H2O) = 36 grams (18 grams × 2)
When analyzing equations we speak of moles so as to balance them just like we did earlier. Now, tell me how many grams make two moles of hydrogen?
Finding solutions to the mole problem
Solutions form when substances combine. Solutions contain solutes. When two
solutions are mixed, the number of moles of solute in one solution exists in a ratio to the number of moles of solute in the other solution. This ratio of solute in solution relative to a specific volume of the solution is referred to as its concentration. Concentration is measured in moles per cubic decimeter or moldm-3. What this simply means is that you find an estimate of the number of moles of the solute present in each decimeter cube of the solution. 1 dm-3 is equal to 1000 cm3 which is equivalent to 1 litre. It is important that you note the following formula to aid calculations in this scope.
Concentration (moldm-3) = amount of solute (mol)... (8)
Volume ( dm3)
“A solute is a substance that is dissolved in a solvent
to form a solution.”
“A solvent is a substance that dissolves a solute to
form a solution.”
35
EXAMPLES
Let’s play around with some problems so you familiarize yourself with the concepts.
1. Write down the masses occupied by 6.02 × 1023 atoms of the following elements: a. Ca
b. Cu
c. N2
d. O
e. H2
Solution
a. 1 mole of a substance contains 6.02 × 1023 elementary entities.
1 mole of an element is equivalent to the atomic number of the element in grams.
The mass occupied by 6.02 × 1023 elementary entities of Ca is therefore 40
grams (or 40 g )
*Consult the periodic table to confirm these atomic numbers.
b. 1 mole of a substance contains 6.02 × 1023 elementary entities.
1 mole of an element is equivalent to the atomic number of the element in grams.
The mass occupied by 6.02 × 1023 elementary entities of Cu is therefore 63.55
grams (or 63.55g )
c. 1 mole of a substance contains 6.02 × 1023 elementary entities.
1 mole of an element is equivalent to the atomic number of the element in grams.
The mass occupied by 6.02 × 1023 elementary entities of N2 is therefore 28
grams (or 28 g )
I imagine you’re wondering what’s even going on. Perhaps you had checked the
periodic table and saw a 14 for nitrogen. This is because 14 is the atomic
number for nitrogen, meaning the number of protons or electrons in an atom of an element. Nitrogen however shown here is N2. It is a molecule of nitrogen (in its stable state) with 2 atoms. So we know that we have to add the 14 of the first atom to the 14 of the second atom to deduce the overall atomic number of the
molecule.
N + N → N2
14 + 14 = 28
36
d. 1 mole of a substance contains 6.02 × 1023 elementary entities.
1 mole of an element is equivalent to the atomic number of the element in grams.
The mass occupied by 6.02 × 1023 elementary entities of O is therefore 16 grams
(or 16g )
e. Refer to (c.)
1 mole of a substance contains 6.02 × 1023 elementary entities.
1 mole of an element is equivalent to the atomic number of the element in grams.
The mass occupied by 6.02 × 1023 elementary entities of H2 is therefore 2 grams
(or 2 g )
2. What is the mass of 0.5 moles of CO2?
When faced with questions of this nature, you find the relative molecular mass
of the compound by adding the masses (atomic masses) of the individual atoms.
Solution
C O O
Individual masses: 12 + 16 + 16
Total mass: 44 grams
Since 1 mole of a substance is equivalent to the atomic number of an element or the relative molecular mass of a compound; 1 mole of CO2 is 44 grams.
If 1 mole = 44 grams
Then 0.5 moles = x
X = 44 grams × 0.5 moles
= 22 grams (or 22g)
3. Balance the following equation:
C2H6 + O2 → CO2 + H2O
Let’s begin by looking at each element on both sides of the equation. Begin with C. There are two carbon atoms on the reactant side, but only one on the product side. Let’s try to balance this by putting a ‘2’ in front of the C on the product side.
C2H6 + O2 → 2CO2 + H2O
Now we have two carbon on each side. However, oxygen is now 2 on the
reactant side and 5 on the product side (multiply the 2 in front of CO2 as it includes oxygen by the subscript 2 beside oxygen and add those to the single O
37
in H2O.) We see that the equation is still not balanced. This just goes to show that you may require more than one try to balance an equation.
Now let’s try something else. Let’s begin with H instead. H is 6 on the reactant side so we add 6 in front of H on the product side. This has made the product H
now 12 and the reactant H remains 6.
C2H6 + O2 → CO2 + 6H2O
Then we balance O and C. Product O is now (6 + 2) which is 8. We can put a 4 in front of reactant O but the equation won’t be balanced. Instead let’s put a 4 in front of product C.
C2H6 + O2 → 4CO2 + 6H2O
Product O has become 14. We can add a 7 to reactant O to balance O.
C2H6 + 7O2 → 4CO2 + 6H2O
What about C? It is 4 on the product side and 2 on the reactant side. We now
add a 2 to C on the reactant side and it becomes balanced.
2C2H6 + 7O2 → 4CO2 + 6H2O
As you can see, H is also balanced on both sides. Take a careful look at the
equation. You will notice that all the elements are balanced on both sides.
4. How many moles of calcium chloride (CaCl2) are there in 1 dm3 of 2.0 moldm-3
of solution?
Solution
Applying, the initial equation;
Concentration (moldm-3) = amount of solute (mol)
Volume ( dm3)
Volume = 1dm3
(It is imperative that we convert the volume to dm3 but in this case we don’t have to.) Concentration = 2.0 moldm-3
Amount of solute in moles (also known as number of moles of solute) = X (unknown) Make X (amount of solute) the subject of the equation;
38
Amount of solute (mol) = Concentration (moldm-3) × Volume (dm3)
X = 2.0 (moldm-3) × 1 (dm3)
X = 2 moles.
NOTE: Many students of chemistry, mathematics and the other sciences have adopted a bad habit of neglecting standard units in their calculations such as kg, g, moldm-3 or
cm3. It is easy to fall into that negligence trap but please don’t. I know I did that for a while until I realized my mistake, after which it caused me some good marks and more importantly some good learning. You need to understand that the S.I units are as involved in the calculations as the numbers themselves. For instance, take a look at the equation above:
X = 2.0 (moldm-3) × 1 (dm3)
Let’s spread this wide a bit;
X = 2 × moles × 1 × dm3
dm3
Now we divide;
X = 2 × moles × 1 × dm3
dm3
X = 2 × moles × 1
X = 2 moles.
Isn’t that clearer? Don’t forget this the next time you solve any equations.
Empirical, Molecular and Structural Formulas
We have come across the term ‘relative molecular mass’ before. We were trying to
calculate the mass of compounds like H2O by adding the individual masses of the
atoms that make up the compound. The relative molecular mass is not only useful in calculating masses but also aids scientists in finding out what the possible formulas of compounds are. In some cases chemists are given a certain material that contains a number of elements but have no idea in what ratio these elements are combined or what the arrangement of the atoms in each molecule are. In such cases they carry out experiments to find out the masses of the individual elements and then are faced with the problem of finding out what the formula of that compound to be. Two kinds of
formulas are obtained from this analysis. These are the empirical and molecular 39
formulas. A formula that can also be obtained is the structural formula but this will be thoroughly dealt with later.
“An empirical formula is a formula that shows the
simplest ratio of elements that are present in a
molecule of a compound but does not show the
specific number of atoms in a molecule of the
compound or arrangements of the elements in a
molecule of that compound.”
A bit of a mouthful, but all it really says is that empirical formulas give you suggestions of how much of each element is present in the compound but can’t tell you the exact number of atoms in one molecule or in what way they are arranged. For instance with just an empirical formula, how can I tell if ‘C’ should be before ‘O’ in CO2? What if it’s the other way around?
“A molecular formula gives the total number of
atoms present in each molecule of a compound.”
The molecular formula is more exact. It shows the specific number of atoms in one molecule of a compound.
“A Structural formula is a formula that shows how
atoms are arranged in a molecule of a compound.”
As stated earlier, this would be dealt with later. You would certainly want to stick around because it is literally a beautiful subject.
EXAMPLES
5.6 g of a compound containing carbon, hydrogen and oxygen on combustion gave 7.2g of CO2 and 4.5 g of H2O. The relative molecular mass of the compound was found to be 62.
a) What are the masses of C, H and O are contained in 5.6g of this compound?
40
b) What are the simplest (empirical) and molecular formulas of the compound?
Solution
a) Let’s begin with carbon.
Mass of unknown compound = 5.6g
Mass of CO2 given off on combustion = 7.2g
7.2
Mass of CO2 relative to mass of compound =
= 1.286g
5.6
12
Mass of C in CO2 =
= 0.273g
44
Percentage of C in compound = Mass of CO2 relative to mass of compound × Mass
of C in CO2 × 100%
= 1.286 × 0.273 × 100%
= 35.0%
Percentage of C in compound is therefore 35%.
Mass of C in the compound is gotten by the following;
X
35% =
× 100%
5.6
Where X is the mass of C in the compound.
196 = 100X
X = 1.96g
The mass of C in the compound is 1.96g.
Now we find the mass of Hydrogen.
Mass of unknown compound = 5.6g
Mass of H2O produced on combustion = 4.5g
4.5
Mass of H2O relative to mass of compound =
= 0.804g
5.6
2
Mass of H in H2O=
= 0.111g
18
Percentage of H in compound = Mass of H2O relative to mass of compound ×
Mass of H in H2O × 100%
= 0.804 × 0.111 × 100%
= 8.9%
Percentage of H in compound is therefore 8.9%.
Mass of H in the compound is gotten by the following;
Y
8.9% =
× 100%
5.6
Where Y is the mass of H in the compound.
49.84 = 100Y
Y = 0.50g
The mass of H in the compound is 0.50g.
We don’t need to repeat the entire process for oxygen since we know the
percentages occupied by the other two elements. Percentage mass of oxygen is
then:
100% - (% mass of C + % mass of H)
= 100% - (35.0% + 8.9%)
= 100% - 43.9%
= 56.1%
41
To find the mass of oxygen in the compound, we repeat what we’ve done with the other two:
Z
56.1% =
× 100%
5.6
Where Z is the mass of O in the compound.
314.16 = 100Z
Z = 3.14g
The mass of O in 5.6g of the compound is 3.14g
In order to confirm this, you can add all three masses together and see if the result is 5.6g. When you do, you’ll find that it is exactly 5.6g and in that case we are correct.
b) In order to obtain the empirical formula, we begin by finding out how many moles of the atoms are present in the masses. For instance how many moles of C
atoms are there in the 0.96g of carbon in the unknown compound? We can know
this by dividing the masses by their respective molar masses (masses occupied by 1 mole).
C : H : O
1.96
0.50
3.14
∶
∶
12
1
16
0.163 ∶ 0.500 ∶ 0.196
What we do next, is divide these numbers by the number of least magnitude or the
most occurring. In this case the most occurring is 0.163.
0.163
0.500
0.196
∶
∶
0.163
0.163
0.163
1 ∶ 3 ∶ 1
C : H3
: O
The ratio of the constituents is 1:3:1 so the empirical formula becomes CH3O.
The molecular formula makes use of the Molecular mass and the Empirical Formula
Mass. The empirical formula mass can be gotten in the following way:
CH3O = 12 + (3 × 1) + 16
= 12 + 3 + 16
= 31g
You then divide the molecular mass given by the empirical formula mass to obtain a special number n. The molecular mass in this case is 62.
Molecular Mass
n =
Empirical Formula Mass
62
n =
31
n = 2.
Finally, we multiply n by the ratios of the empirical formula. The molecular formula thus becomes;
(CH3O)n
=(CH3O) 2
= C2H6O2
This final result is the molecular formula of the compound
42
43
. SUMMARY:
An atom is the smallest unit of a substance that can take part in a
chemical reaction.
A molecule is a group composed of two or more chemically bonded
atoms.
Elements are pure chemical substances that are composed of atoms
of the same kind.
Compounds are composed of two or more chemically bonded
elements.
A solute is a substance that is dissolved in a solvent to form a solution,
while a solvent dissolves a solute to form a solution.
A solution is a homogenous mixture of substances (solutes) in which
the constituents (atoms, molecules and ions) of the substance are
dispersed completely in a solvent.
The periodic table consists of all the known elements and their atomic
numbers.
A chemical equation is a written representation of a chemical reaction.
Reactants are substances that undergo a chemical reaction to give
products and are found on the left side of the equation.
Products are substances formed when reactants undergo a chemical
reaction. They are found on the right side of the equation.
A chemical reaction is a process that involves the conversion of one or
more reactants to yield one or more products.
Elements and compounds show significant differences (check the
MNEMONICS section of this chapter).
An unbalanced chemical equation is not a pretty sight to a chemist.
Avogadro’s law states that equal volumes of all gases at the same
temperature and pressure contain the same number of molecules.
A mole is an amount of a substance that contains as many elementary
entities or units as they are in 12g of the carbon-12 isotope.
An empirical formula shows the simplest ratio of elements present in a
molecule of a compound.
A molecular formula shows the total number of atoms present in a
molecule of a compound.
44
The structural formula of a compound shows the arrangement of the atoms in a molecule of the compound.
45
MNEMONICS
Here we would be attempting to memorize the differences between elements and
compounds.
T
B
N
S
(F)
T – Types: As in “Elements are composed of one type of atom, while compounds are not.”
B – Broken down: As in “Elements cannot be broken down into simpler forms but compounds can.”
N – Number: As in “They are a fixed number of known elements in existence, but the number of compounds are almost limitless.”
S – Symbols: As in “Elements are represented by symbols but compounds are
represented by chemical formulas.”
The ‘F’ stands for formulas and is optional.
Can you think up interesting mnemonics with this? How about; ‘Tall Bats Never See (Far)’? or maybe ‘Tiny Bosons are Nature’s Symbols (and Formulas)’ ? That has got something to do with atoms at least. Think up creative mnemonics so you never forget them.
46
REVISON QUESTIONS
1. Define the following terms:
a) An atom
b) A molecule
c) An element
d) A compound
e) A solution
f) Atomic number
g) Mass number
h) Solute
i) Solvent
j) A mole
2. Chlorine has two well known isotopes which are chlorine-35 and chlorine-37.
How many protons, electrons and neutrons does each isotope possess?
3. State four differences between elements and compounds.
4. Balance the equations below:
a) CO2 + H2O → C6H12O6 + O2
b) KClO3 → KClO4 + KCl
c) Fe2O3 + C → Fe + CO2
d) Al + HCl → AlCl3 + H2
e) FeCl3 + MgO → Fe2O3 + MgCl2
5. Write down the masses occupied by 6.02 × 1023 atomsof the following elements: a) Mg
b) Na
c) O
d) Be
e) S
6. Write down the masses occupied by 6.02 × 1023 moleculesof the following
elements:
a) F2
b) O2
c) P4
7. What are the number of atoms in;
a) 0.5 moles of H2O
47
b) 12g of Mg
c) 2 moles of NaOH
d) 6.5g of Ca
e) 0.1 moles of O2
8. How many moles of sodium iodide (NaI) are there in 1000cm3 of 3.0 moldm-3 of
solution?
9. 6g of an element X with a molecular mass of 40 reactedwith 12g of chlorine to form a chloride.
a) How many moles of chlorine combined with one mole of X?
b) What is the simplest formula of the chloride?
10. Is it true that equal volumes of all gases weigh the same? Give reasons for your answer.
48












