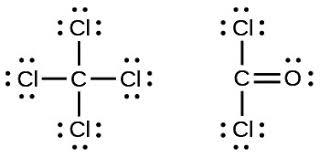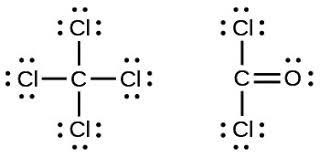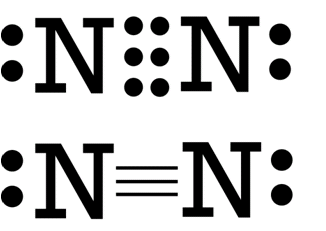CHAPTER 3: CONFIGURATIONS AND
CHEMICAL BONDS
Imagine a universe where atoms are rigidly unsociable and never meet other atoms for tea, or to walk their electron pets or dance to Maroon 5 or Korede Bello. How such a bore that would be! There would be no working together so they’ll be little development.
Structures won’t be formed and that dear friend, means you and I won’t even be here.
Atoms on their own are fascinating building blocks, but in the absence of combining with other atoms they build nothing. All the beautiful things around us have atoms that make them up. The very biological processes we undergo are as a result of the atoms and molecules that make up our bodies. You and I are physical beings and can feel the air, touch random strangers (don’t do it, believe me I know), feel pain when we suddenly strike our feet on surfaces, run our fingers through running water and slap that annoying cousin we see every Christmas (also, don’t do it). We can do all this because atoms have chosen to be friendly with each other; because they have decided to create
bonds.
Figure 3-1Everything is made from atoms because atoms bond.
In fact, they have to create bonds. Just like many adults and almost all kids atoms have a hard time sitting still. I can’t blame them; I have a hard time sitting still. There’s always 49
so much to do, and even writing this book is an example. The reason for this is that just like how the very point of life for many people is to be mentally, physically, emotionally and financially stable and happy, atoms also want to be stable and happy. Okay, maybe not ‘happy’ but they want to be stable nonetheless. Not sure what this stability means?
Hang on a bit and find out.
The Octet Rule
If there was one thing in the world that could make all your dreams come true and you knew how to get it, wouldn’t you go for it? I’ve got no supernatural powers (unless you count not sleeping) but I’m quite sure you said yes. Also, just on a friendly note, no matter how stressful school and work get, make sure you get some sleep; always.
Atoms have figured their key for ultimate stability and it is called the octet rule. Atoms here are trying to balance the number of electrons on their shells by getting at least close to 8 electrons on their outermost shells (hence the name ‘octet’) in imitation of the noble gasses.
Noble gasses are found on the farthest column to the right of the periodic table. You can look them up. Elements found there are helium (He), Neon (Ne), argon (Ar), Krypton (Ke), Xenon (Xe), Radon (Rn) and Oganesson (Og). An interesting fact to know is that Oganesson was discovered only in as recently as 2002. That may not seem so recent but many chemistry texts have not included it in this list. I didn’t learn about it in school but I’m glad you can.
The noble gases listed above are the popular guys of the periodic table kingdom.
Everyone else wants to be them because they are so chill, nothing seems to bother them. The other elements want a taste of that cool. The only to achieve that is to have at least a near octet outer shell. Let’s find out how to go about helping the elements get cool with some help from Gilbert N. Lewis. Walk with me again.
50

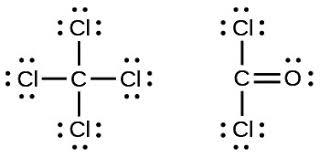
Figure 3-2 Gilbert N. Lewis working.
It is the year 1916 and we find the American scientist on his desk writing extensively an article titled: The Atom and the Molecule. I sit on a chair and nearly fall to the floor dozing off (Wow. I need to sleep) while you bend over the shoulders of the scientist and find strange diagrams just like the ones below:
Figure 3-3 A Lewis dot structure.
What could these weird dots and dashes possibly be? The dashes and dots are valence electrons.
“Valence electrons are the electrons located on the
outermost shell of an atom.”
Carefully study the diagram below.
51
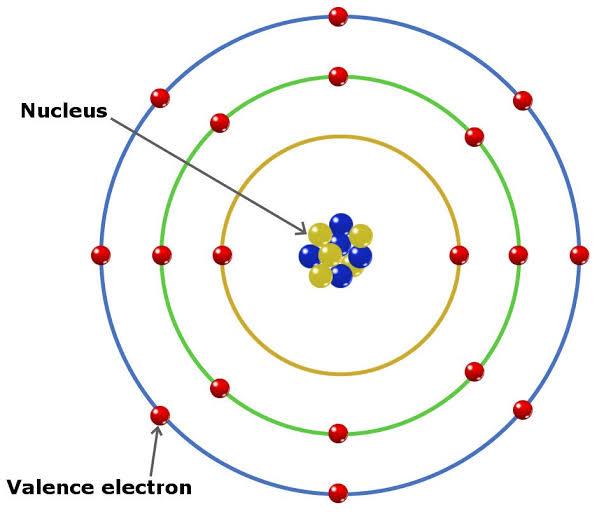
Figure 3-4 Atomic structure showing the nucleus and valence electrons.
All those concentric circles you see above are orbitals or shells of an atom. Whenever an atom interacts with other atoms, the valence electrons on these valence shells are the first to participate in the reaction.
Picture this. Imagine you are seated in a classroom with the rest of your classmates for a chemistry lesson (just a randomly chosen subject) and there is only one door to the classroom located at the back of it. None of you hold on to anything and never do for the duration of that class. You all also roughly weigh the same. If a sentient gigantic vacuum cleaner paid a surprise visit to your class and standing at the door began sucking up all of you, based on the location of the door which students would be the first to be sucked in? The ones seated in front or the ones at the back? I’m guessing you said the ones at the back. If so, congratulations! Here’s a cookie.
Just like your classroom, the electrons situated farthest from the nucleus in the middle of the atom would be the ones to participate when other atoms show up to engage the atom.
But wait. There’s more. The electrons located at the farthest shell of the atoms are there on account of something known as the screening effect. The electrons are negatively charged. The protons in the nucleus are positively charged. Remember when we
mentioned that negative and positive charges attract each other? Similar charges on the other hand repel each other. So when more than one electron exists in an atom, all the electrons exhibit what is known as a screening effect. This means that they screen each other or in simpler terms refrain from ‘touching’ each other on account of the fact that 52
they possess the same charge and as we know, their negative charges should and do repel each other. What this means is that the screening allows for greater distance between them, and naturally some electrons are pushed to the valence shell.
But how do we know them? If I were asked to say how many valance electrons oxygen had would I be able to? I just may so now let’s get you to do so too.
Deducing the number of valence electrons of atoms
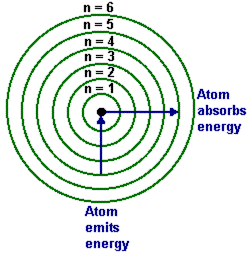
In order to easily visualize how electrons are arranged in an atom and work with atoms, the shells are labeled K,L,M,N and it continues. Each shell is given the number n, known as the principal quantum number where n is1, 2, 3 and so on depending on the location of the shell, so that the shell closest to the nucleus is given n = 1 and the second shell is n = 2 and on it goes.
The numbers are important because they can be inputed into the formula 2n2 which helps to show how many electrons are in a particular shell. If your memory is really sharp, I’m quite sure an old friend visited your thoughts, and this image from chapter one crossed your mind:
Figure 3-5 Niels Bohr’s atomic depiction showing the electron shells.
That’s right. The idea of electronic configuration which shows how electrons are arranged in atoms is the brain child of our old friend, Niels Bohr. Let’s add a little thing in the light of new information.
53
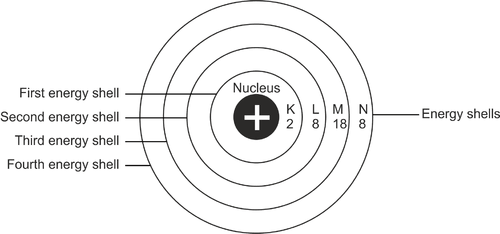
Figure 3-6The electron shells labeled.
The first shell is the K shell meaning n=1. In order to know how many electrons occupy that shell, we use the equation 2n2. When we substitute n for 1 as in;
2n2 = 2 × (1)2
= 2 electrons.
We therefore now know that the K shell occupies 2 electrons and can only occupy 2
electrons. What about the L shell? n for the L shell is 2 so the number of electrons on the shell are 8. Can you find out how many electrons are on the M and N shells? I’m sure you can. I’ll wait.
It’s about time for some real world examples or as real as the mysterious atoms can get.
Let’s try deducing the arrangement of electrons on say, the oxygen atom. Oxygen has an atomic number of 8 and a mass number of 16. Right now our focus is on the atomic number. An atomic number 8 means that the number of protons are 8 but if you have been following carefully, you would remember that atomic number is not just the number of protons, but the number of electrons as well because in a neutral atom the number of electrons are protons are equal. What this implies is that the number of electrons in an oxygen atom are also 8. Let’s try arranging these 8 electrons on the shells of our oxygen atom.
We can start with the K shell. We have already established that the K shell has a maximum of 2 electrons, following the 2n2 formula and that the L shell has a maximum of 8 electrons. How many shells do you think oxygen has then? How would you arrange the electrons on the shells and how many valence electrons does it have? I’ll wait.
54
Asking where I went to? Oh I took a well-deserved break because teaching is hard. Not that I don’t love it of course. I also had a cold drink. You could get yourself one. You look like you need your own well deserved break. Done? Let’s begin.
Before the break I had left you with a small problem. Did you find the answer? I’m sure you did. Let’s solve it together.
Oxygen has atomic number 8. This means it has 8 protons and also 8 electrons. The maximum number of electrons that can fill a K shell is 2 electrons, while the maximum number capable of filling an L shell is 8 electrons based on the formula 2n2. Since 2 + 6
is 8, the number of shells required for the oxygen atom are the first two shells; K and L.
As for the arrangement, you might want to get a pencil. Look at the diagram below.
55
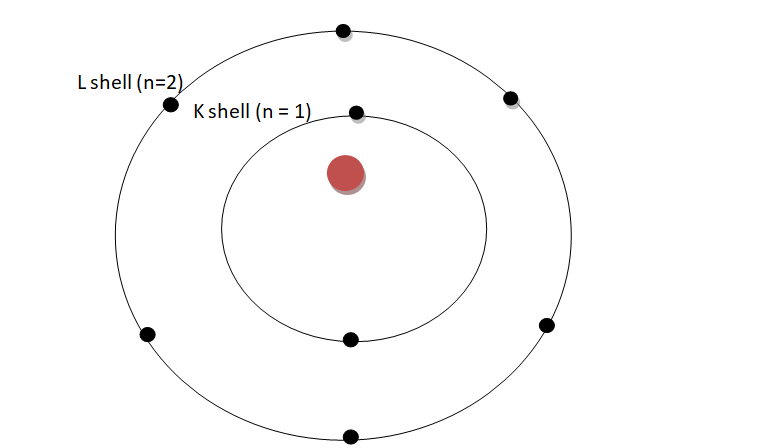
Figure 3-7 Electron shell diagram for oxygen.
As you can see above, the K shell has two electrons while the L shell has 6 electrons.
The red oval in the centre represents the nucleus. From the diagram we can see that oxygen’s valence shell is the L shell. Therefore oxygen has 6 valence electrons. Try drawing the shell diagram for the element carbon with atomic number 6. How many
valence electrons does it have?
Met the shells? Say hello to the sub-shells
I am almost standing on my sit writing about these principal shells (as they are called) we’ve talked about. This seems to be the first time in a while we’ve talked about something relatively simple. I mean, all we’ve got to do is know an element’s atomic number and magic! We could know the number of its valence electrons, what it may
look like and possibly even what it had for dinner. That’s great and suspiciously easy.
From what we’ve known about the atom it loves to play jack-in-the-box and any moment now it’ll pop up with a surprise. You probably already guessed it. Yes, the shells aren’t the only thing to remember, we will now talk about sub-shells and I think you probably don’t get why they matter because I didn’t get it myself for quite a long time.
They are the subshells of the principal shells denoted by the spdf (and so on) notation.
While in the principal shells we spoke of the principal quantum number n, with subshells we talk about the azimuthal quantum number ℓ, where ℓ is 0,1, 2, 3 and so on depending on the subshell. Hence if the subshell is d, ℓ is 2. This system is analogous to 56
a desk. There is only one desk, but there are a number of drawers that can be used to store multiple things. In the same way the principal shell K can be further divided into the subshells s, p, d, f and so on depending on how many electrons need to be ‘put away’ in this subshell. The maximum number of electrons that can be found in a
subshell can be gotten by the formula;
2(2ℓ + 1)
Let’s summarize this briefly so your mind doesn’t become as cluttered as my desk.
There are a number of principal shells denoted by K, L, M, N and so on. These shells show how electrons are arranged in the atom. This arrangement is obtained by
harnessing the equation 2n2 where n is the principal quantum number represented by 1, 2, 3, and on depending on the location of the shell relative to the nucleus. The shells are further divided into subshells represented by s, p, d, f and on it goes. These subshells show how electrons are arranged in them using the azimuthal quantum
number ℓ, which can be can be 0, 1, 2, 3… depending on the subshell so that for the p subshell it is 1. The maximum number of electrons that can occupy a subshell is
calculated using the formula 2(ℓ + 1).
Writing the electronic configuration
We have talked about drawing shells. That seemed nice but could you imagine having to draw that for an atom with atomic number 118? How wide would your paper have to be? This is no joke though, 118 is the atomic number for organesson, the new addition to the periodic family. In order to be merciful to us, not just because it’ll take a good deal of space (and time) to draw such a structure but because not all of us are gifted in drawing just about anything, Niels Bohr and other scientists introduced a more terrible artist friendly way to represent the arrangement of electrons in orbitals.
For example, we can talk about 3d. Not the viewing option 3D but the electronic configuration 3d. What this means is that the electron(s) here is/are on the third principal shell represented by the ‘3’ and the d subshell. If my missing electron is on 4s?
What does this mean? I’ll wait.
Yes, that means it’s on the N principal shell (the 4th principal shell) and the s subshell.
If I wanted to represent more than a few electrons but wanted to show all the electrons of one atom, then I’ll have to put them all together. For example if you are asked to write the electronic configuration of an atom of chlorine (Cl) with atomic number 17, you would need to put all those shells together. The atomic number of chlorine would then become:
57
1s2, 2s2, 2p6, 3s2, 3p5
If you add up all those electrons shown by the superscripts you would get 17, the atomic number of chlorine. Following the formula 2(2ℓ + 1), you would find that the maximum number of electrons in the s subshell is 2 and in the p is 6. Follow this, and you’ll be sure to get it right. Let’s try another example for good measure. Let’s try writing the electronic configuration for calcium (Ca) with atomic number 20.
1s2, 2s2, 2p6, 3s2, 3p6, 4s2
Try writing the configurations for magnesium (Mg) and Potassium (K) and get a feel for how fun writing them can be.
Laws that aid electron orbital assignment
Chemistry isn’t a subject for crazy people…relatively speaking. It’s a field that follows rules and this is because the central subject of our studies is one that is dependent on rules as well. When atoms perplex us, it isn’t because they are anarchistic, but it’s mostly because we just don’t understand their rules. However there are some rules we understand and that’s because brilliant men and women have learnt them. Some of
these rules are the Pauli exclusion principle, Hund’s rule and the Aufbau principle and they aid us in knowing the right way to assign electrons to orbitals.
Pauli’s Exclusion Principle
Wolfgang Pauli won the 1945 Nobel prizefor the formulation of the Pauli’s exclusion principle which he formulated in the year 1925. The law states that no two electrons can have the same four quantum numbers. In school I had prided myself on memorizing this law but I never quite knew what it meant. I realize now that it was a waste of time cramming up impressive words. It’s better understanding them, so let’s understand.
“Pauli’s Exclusion Principle states that no two
electrons can have the same four quantum
numbers.”
What this simply implies is that all electrons are unique and as such cannot have the same quantum numbers or occupy the same positions. Examples of these ‘quantum
numbers’ are the principal quantum number and azimuthal quantum number which if
you are keen I’m quite sure that you have noticed that the law mentioned four quantum 58
numbers, whereas we have only discussed those two ( n and ℓ). They are actually more than two quantum numbers, the others being the magnetic quantum number (mℓ) and the electron spin quantum number (ms) but those are not as relevant for our present scope but will be dealt with later.
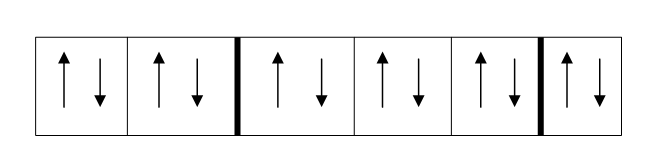
According to Pauli’s law, only two electrons can occupy the same orbital, and if two electrons exist in that orbital they must be of opposite spins. Spins are a form of angular momentum of an electron. At present, just think of them as ‘arrangements’ of electrons where each electron is like an arrow pointing in a particular direction. Pauli’s principle shows that no two of these ‘arrows’ could have the same spin (point in the same direction) in the same orbital.
1S2
2S2
2P6
2S2
Figure 3-8 Atomic number of Mg represented by a spin diagram
Each of the boxes above represents an orbital and the number and letter units above are shells. In each orbital you can see that Pauli’s principle is obeyed.
Hund’s Rule
Friedrich Hund stated this rule in 1925, which concerns how orbitals are filled.
Whenever electrons occupy orbitals, they do so one at a time before filling up the shells.
I’ll explain.
“Hund’s rule states that in filling orbitals, electrons
do so singly first before occupying the orbitals in
pairs.”
What this means is that even when a pair of electrons are needed in an orbital, they fill the orbital singly before doing so doubly. The atomic number for oxygen is 8. Its electronic configuration is therefore 1s2, 2s2, 2p4. When asked to show its spin diagram you may give the diagram below:
59
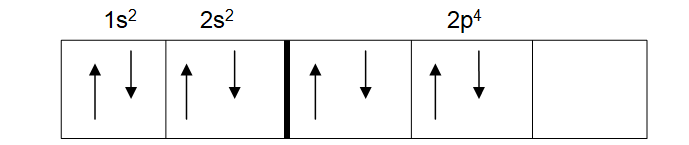
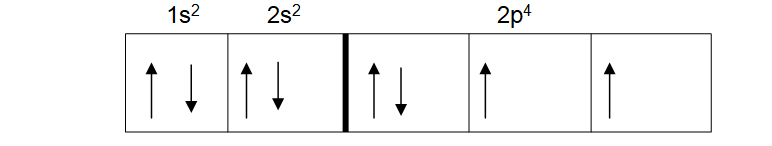
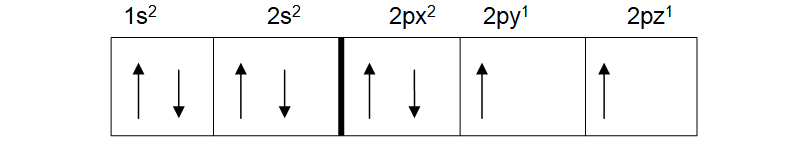
This may seem right to you, but it breaks Hund’s law. This is because the third p orbital is empty. It was meant to be filled first before starting again with the first p orbital. If done correctly, the spin diagram of oxygen should look like the one below.
This obeys Hund’s rule.
In order to show what orbital has what electrons, it is helpful to divide the three orbitals of the p subshell into x, y and z. The first p orbital in the above can then be 2px2, the second 2py1 and the third 2pz1. All still amount to 4 electrons when added together.
The Aufbau Principle
The word aufbau stems from a German word ‘aufbauprinzip’ which is ‘building up
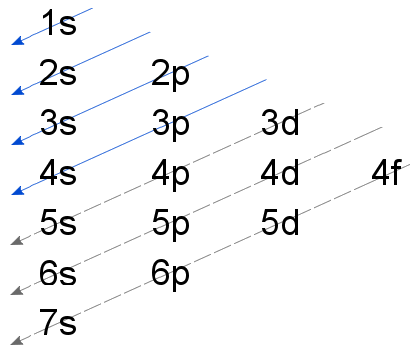
principle’. The principle explains that orbitals can only be filled from the ground state of an atom up. This means that electrons occupy orbitals of lower energies first before moving on to occupy orbitals of higer energy. In the diagram above we see that the 60
lowest energy orbital is 1s, followed by 2s and then 2p, then 3s and so on. The arrows point from higher to lower energy orbitals. When faced with the problem of writing the configurations of atoms with large atomic numbers, resort to this image for guidance.
The image above can be arranged linearly as;
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s and so on.
“The Aufbau principle states that electrons occupy
orbitals starting from those with the lowest energies
to those with the highest energies.”
Now that we know what valence electrons are, shells and electronic configurations are, let’s move on in our journey to deciphering those puzzling dots and dashes of Mr. Lewis and in so doing come to understand chemical bonds.
I can tell you’re exhausted. All this new knowledge came in like a rush.I t’s a good time to set the book down and get a cold drink; fruity flavored if you like and maybe a snack or two. When you return we would be tackling chemical bonds.
Atoms unite! (in chemical bonds)
Wow. We seemed to have left Mr. Lewis by himself so long. Sure hope he isn’t angry but then again he probably has been too preoccupied to care. No problem though. We would get along fine.
61
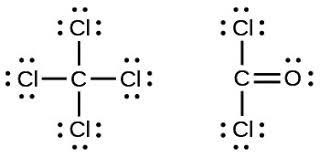
Remember this image? There are the dots and dashes that led us into all that talk about electronic configurations. We should be glad we went through those because
they are going to come in handy. The dots and dashes shown in the picture above
represent electrons but not just any electrons in the atom. They represent valence electrons which you remember are the electrons on the outermost shell of the atom otherwise known as the valence shell.
When atoms interact, their valence electrons take over and are sometimes given
away or accepted. When atoms give their valence electrons away in order to obtain a stable or octet configuration, the type of bond formed by the participant atoms is known as an electrovalent or ionic bond.
“An electrovalent or ionic bond is a type of
chemical bond formed between atoms in which
one atom loses an electron to form a positively
charged ion (a cation) and another atom gains the
electron to form a negatively charged ion (an
anion).”
Remember when we spoke of an ion and said we’ll revisit it at another time? Now is just that time. When electrovalent bonds are formed the valence electrons are
transferred from one atom to the next. Prior to this transfer, both atoms are both relatively neutral. When the atoms interact and form this bond, they change. By
giving away an electron one atom changes because the number of electrons have
reduced and the number of protons are higher than those of electrons, meaning it is 62
now less negatively charged than before and hence more positively charged. The
atom that receives the electron however now has an increased number of electrons, more so than the number of protons and so is more negatively charged than before.
When an atom loses an electron it is no longer neutral and becomes an ion. This ion is more positive than before and so we call it a cation. As for the atom that received the electron, it is also no longer neutral and so is an ion. Since this ion has received an electron and become more negative than before, it is now an anion.
“An ion is an electrically charged atom or molecule
formed by the loss or gain of an electron.”
“A cation is a positively charged ion.”
“An anion is a negatively charged ion.”
By a transfer of electrons hence, electrovalent or ionic bonds are formed. A good example of a compound formed via ionic combination is sodium chloride (NaCl)
which you know more familiarly as table salt.
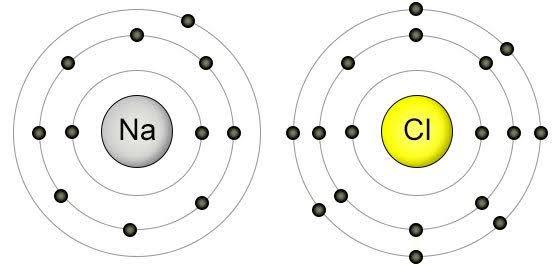
Sodium (Na) has the atomic number 11 while chlorine (Cl) has atomic number 17.
The principal shells of Na using the form K,L,M,N can be written as 2,8,1 where
there are 2 atoms in the K shell, 4 in the L shell and so on.
For Cl with atomic number 17, its configuration is 2,8,7.
Figure 3-9 Na and Cl dot structures
63
If you observe, Na needs to lose its 1 electron hanging on the outermost shell in order to achieve an octet configuration. Cl on the other hand needs 1 electron to achieve its own octet configuration. Na is therefore generous and gives its electron away and everyone is happy. An octet configuration simply means that just like the noble gasses, there are now 8 valence electrons on the valence shell of the atom, hence “octet”.
When Na gives away its electron, it has a net positive charge as explained earlier.
Being more positively charged, it becomes a cation. Cl, in accepting the electron from Na now has a net negative charge. The two bond and tasty food is born thanks to the salt formed.
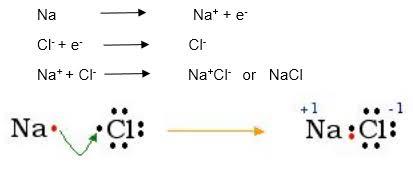
This process can be represented by a Lewis dot structure.
Figure 3-10
The above is a concise representation of the process. The first equation shows Na losing an electron (e-) to become a cation, while the second shows Cl gaining an
electron to become an anion. The final equation shows the entire process. This is an example of how to write an ionic equation. We would write way more of this kind of equation as we move along.
Covalent Bonds
A second type of bond is not concerned with transferring electrons, but has a more
‘altruistic’ approach. This bond is formed when electrons are shared between
participating atoms.
“Covalent bonds are formed when atoms involved
in a reaction share pairs of electrons.”
Three types of this kind of bonds exist; single covalent bonds, double covalent bonds and triple covalent bonds.
64
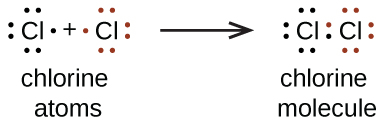
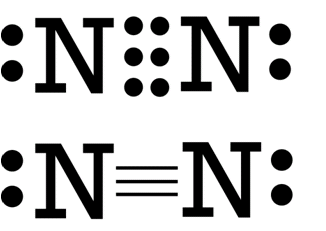
The single covalent bond occurs when a single pair of electrons is shared by the participating atoms. An example is the bond formed in the formation of the chlorine molecule Cl2.
The chlorine atoms require an atom each to complete their octet configuration. In sharing a single pair of electrons, they achieve this and become stable.
The double covalent bond is formed between atoms that share two pairs of
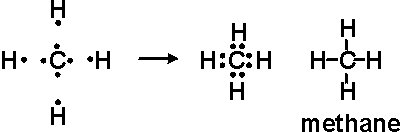
electrons. For instance the bond found in a methane molecule (CH4) when C and H
combine is a double covalent bond.
Each molecule of H contributes one electron to share with the C. All four valence electrons of the H taken together is equal to four electron pairs combining with the four pairs of valence electrons of the C making the bond formed a double covalent bond.
The last type of covalent bond we would talk about is the triple covalent bond. I’m sure you can already deduce what its definition is, but I’ll put it down anyway. This bond is formed when three pairs of electrons are shared by participating atoms in bond formation. Nitrogen gas (N2) is formed in this way.
As you can see, three pairs of electrons are shared by the nitrogen atoms. You may also notice that aside from dots, dashes are also used. As shown above dashes
65
could be used as a substitute for dots, where each dash corresponds to two dots or two electrons.
Properties of electrovalent compounds
The distinctness of an ionic compound doesn’t cease when it is formed. The way an electrovalent compound is bonded plays a large part in the characteristics it exhibits.
The following features are found in compounds formed from an ionic or electrovalent bond.
1. Electrovalent compounds are hard: The ordered arrangement of ions in a
crystal lattice and strong attraction between these oppositely charged ions
has made the structure of an ionic compound well defined and hard. An
example is in table salt (NaCl) which has such crystalline appearance and
cannot be distorted so much as to alter its shape.
2. In the solid state, they are poor conductors of electricity: This however changes when they are dissolved in solution and their ions are free to move
about. NaCl for example can be a great conductor of electricity. You will find
out how in the chapter on electrolysis.
3. They have high melting and boiling points: In simple terms, boiling point is just an indication of what temperature or how quickly something boils when it
is introduced to heat. It is the temperature at which something changes from
liquid to gas. Melting point is the temperature at which something melts away
when introduced to heat, changing from solid to liquid.Pure water has a
boiling point of 100○C. This means that at a 100 degrees celsius, pure water
boils and turns to vapour (which is gaseous) as opposed to helium (He) which
has a boiling point of just -268.9 ○! Unlike He, electrovalent compounds have
high boiling points and melting points too. The reason for this is that the ionic bonds formed between the oppositely charged ions are so strong that it would
take considerable force to dissociate them and alter their states.
4. Ionic compounds are readily soluble in water: When electrovalent
compounds come into contact with water, they dissociate into ions as the
water molecules are attached to them. For this reason they dissolve in water.
66
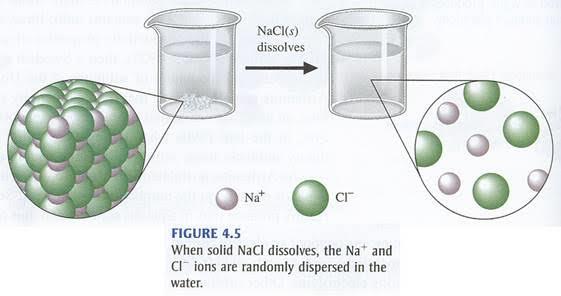
Figure 3-11
Properties of covalent compounds
Irrespective of the type, all covalent compounds share a number of features. These are shown below.
1. Covalent compounds are usually not hard: The electrically neutral
structure of covalent compounds in the absence of strong electrostatic
attraction make them less hard than their electrovalent counterparts. These
compounds are usually liquids and solids on account of this. If solids, they
can easily be deformed because they are held together by weak
intermolecular bonds.
2. They do not conduct electricity: In order for a substance to conduct
electricity, there must be free charged particles available. Ionic compounds
are able to conduct electricity because they produce these particles on
dissociation. This cannot be said for covalent compounds, hence they do not
conduct. A few exceptions however occur in the right conditions. Hydroiodic
acid (HI) and hydrochloric acid (HCl) are these exception. The specific reason
for this would be discussed in a later chapter.
3. They have low melting and boiling points: Having weak intermolecular
forces of attraction explains why they have low melting and boiling points.
They can be easily broken down by heat, changing their states from solids to
liquids and liquids to gasses fairly easily. They are therefore said to be
volatile. An everyday example of this volatility can be seen with kerosene.
You must have noticed the ease with which it dissipates when left in the open.
4. Covalent compounds are not soluble in water: They are soluble in other solvents however. This is because water is a polar solvent while covalent
substances dissolve in solvents that are non-polarsuch as carbon
67
tetrachloride (CCl4) and benzene (C6H6). Don’t fret as we would fully discuss what those terms mean later.
Associate forces of attraction
Aside from those mentioned above, a number of other bonds exist alongside them. For a long time it was believed that there were two distinct bond types (those already mentioned) but scientists are beginning to see that chemical bonding is more or less a spectrum and does not consist of rigidly distinct types. We would briefly discuss each one.
Metallic bond: A good mental image to help us understand metallic bonds is to picture an ‘electron pool’. Atoms seem to ‘swim’ in this pool of mobile electrons forming bonds.
Metallic bonds are similar to covalent bonds in that there is a sharing of electrons, however these electrons are not tightly held to the atoms. Instead they move about from atom to atom in the metal held by the force of attraction between them and the positive ions present. The hardness and conductivity as well as a host of other properties possessed by metals are as a result of the metallic bond.
Hydrogen bond: The hydrogen bond is a relatively weak bond formed between the atoms of hydrogen and those of other elements. These elements are highly
electronegative (meaning that they easily give off their electrons to form cations). Such a bond creates a dipole (charges that are opposite with similar magnitudes). This is because the electronegative atom is partly negative while the hydrogen atom is partly positive as these opposing poles attract, there is the existence of a hydrogen bond.
68
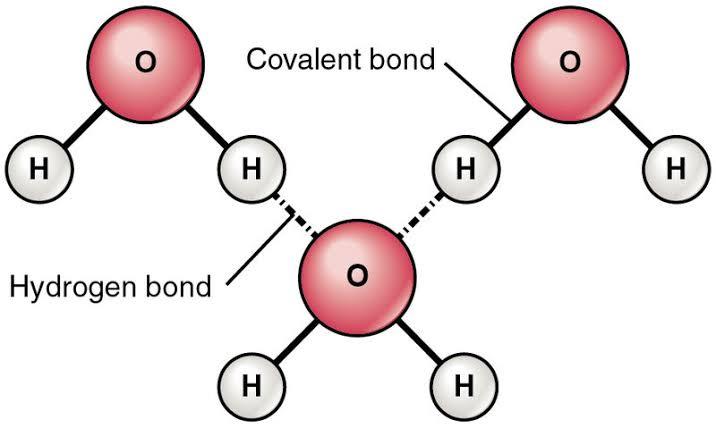
Figure 3-12
Hydrogen bonds are not as strong as covalent and ionic bonds.
Several other forces exist such as the London dispersion force; which is on account of swift dipoles occurring in neighboring atoms. It is a significantly weak force. The cation-pi bond as the name implies, is a force of attraction between a cation and a pi-bond. Van der Waals forces are named after Johannes Diderik Van der Waals who discovered this weak force that is highly dependent on the distance between atoms and molecules. On increasing the distance between atoms, this force breaks down.
Each of these forces are remarkable and can be studied at length. This would be
accomplished at another time, when the need arises.
69
SUMMARY
Atoms are sociable fellows, hence they bond.
The octet rule shows that atoms strive to attain the octet configuration of the noble gases.
Gilbert N. Lewis proposed the Lewis dot structure in 1916 to aid the
understanding of atomic bonds.
Valence electrons are the electrons located on the outermost shell of an atom
known as the valence shell. They participate in chemical interactions that involve the atom.
The screening effect of electrons is a reason why electrons reside in the valence shell of an atom.
K, L, M, N… are known as principal shells with principal quantum number n,
where n is 1,2,3… corresponding to the shell.
The maximum number of electrons on each shell can be obtained by the formula
2n2.
s,p,d,f are subshells of the principal quantum shells with azimuthal quantum
number ℓ, where ℓ is 0,1, 2, 3… depending on the subshell.
The maximum number of electrons on each subshell is gotten by 2(2ℓ + 1).
Writing the electronic configuration is a substitute for drawing shell diagrams.
Pauli’s exclusion principle states that no two electrons can have the same
quantum numbers.
Hund’s rule states that in filling orbitals, electrons do do singly first before occupying the orbitals in pairs.
The Aufbau principle states that electrons occupy orbitals starting from those with the lowest energies to those with the highest energies.
An electrovalent or ionic bond is a type of chemical bond formed between atoms in which one atom loses an electron to form a positively charged ion (a cation)
and another atom gains the electron to form a negatively charged ion (an anion).
An ion is an electrically charged atom or molecule formed by the loss or gain of an electron.
A cation is a positively charged ion while an anion is a negatively charged ion.
Covalent bonds are formed when atoms involved in a reaction share pairs of
electrons.
70
Three types of covalent bonds are single, double and triple covalent bonds.
Electrovalent and covalent compounds differ in their properties (refer to
MNEMONICS after this section).
Other forces of attraction such as metallic, hydrogen, London dispersion, cation-pi and Van dar Waals bonds exist.
71
72
MNEMONICS
Okay. Here we go.
Properties of electrovalent and covalent compounds:
H
E
B
S
H – Hard: As in “Electrovalent compounds are hard but covalent compounds are not.”
E – Electricity: As in “Electrovalent compounds conduct electricity but covalent compounds do not (with a few specific exceptions).”
B – Boiling point: As in “Ionic compounds have high boiling and melting points but covalent compounds have low melting and boiling points.”
S – Solubility: As in “Ionic or electrovalent compounds are soluble in water and other polar solvents but covalent compounds are not soluble in water and polar solvents but in non-polar solvents.”
Can you think up interesting mnemonics with this? How about just ‘HEBS’? (similar to herbs) or maybe ‘Happy Eels Buy Ships’ ? Maybe ‘High Electricity Blows Sockets’.
Think up creative mnemonics so you never forget.
Types of chemical bonds:
L
M
C
V C H I
L – London dispersion forces
M – Metallic bonds
C – Covalent bonds
V – Van der Waals forces
C – Cation-pi bonds
H – Hydrogen bonds
I – Ionic bonds
Can you think up interesting mnemonics with this? How about ‘London May Choose
Very Heavy Ink’? Think of interesting mnemonics so you don’t forget.
73
REVISION QUESTIONS
1. State the following laws:
a) Hund’s rule
b) Pauli’s exclusion principle
c) The Aufbau principle
2. a) What are valence electrons and valence shells?
b) Draw electron shell diagrams for the following atoms:
i.
O
ii.
N
iii.
Be
iv.
H
v.
Si
c) How many valence electrons do each of them possess?
d) Write down their electronic configurations.
3. Briefly explain the following:
a) Electrovalent bonds
b) Ionic bonds
c) Covalent bonds
d) Anions
e) Cations
4. In a tabular form list the differences between electrovalent and ionic
compounds.
5. What type of bond can be found in the following compounds and molecules:
a) H2O
b) N2
c) I2
d) NH3
e) CH4
6. Draw spin diagrams for N, O and F.
7. List and explain seven types of chemical bonds.
8. [Ar] 3d10 4s1
a) What atom has the configuration above?
74
b) How many valence electrons does it possess? (If you get this, you’re a budding Einstein. Put on your thinking cap and give it a try).
9. Why do atoms attain stability?
10. Write the electronic configuration for oganesson (Og) with atomic number
118. (Don’t cry. This isn’t a compulsory one but it helps so you practice).
75