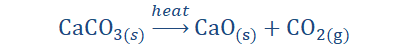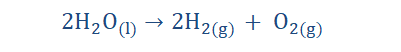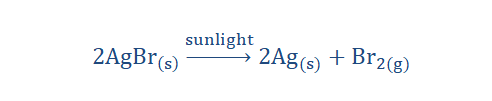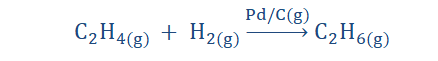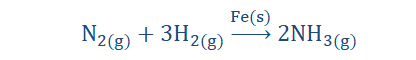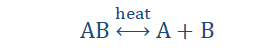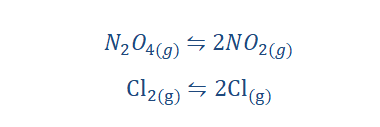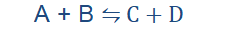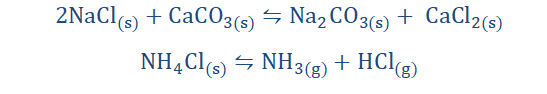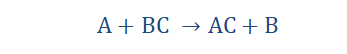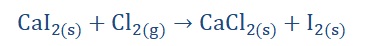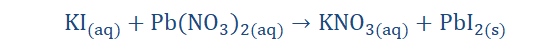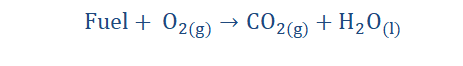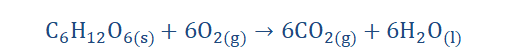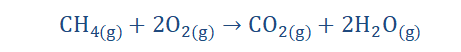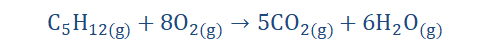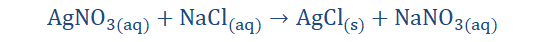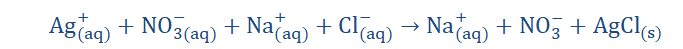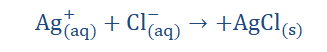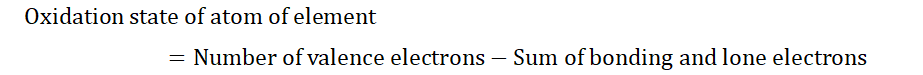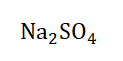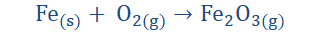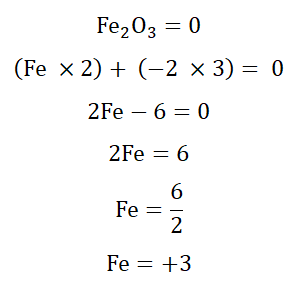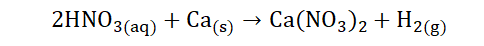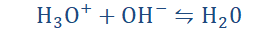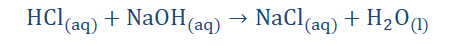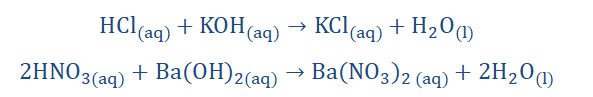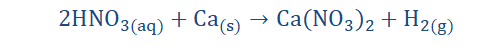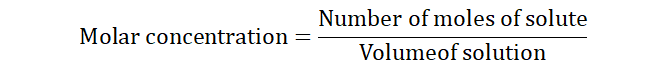CHAPTER 5: REACTIONS
Kinetics and Types
We had briefly introduced the concept of a chemical reaction in chapter 2. There we defined what chemical reactions are and examined a few examples of those. In this chapter we would go a bit further by discussing the different types of reactions that there are as well as their kinetics.
“Chemical kinetics is a branch of chemistry that
deals with the study of the rates of chemical
reactions.”
A lot of exciting things occur when a chemical reaction takes place. The atoms of the reactants interact in particular ways that lead to the formation of the products. You see the results of those interactions through color changes, subtle weathering,
explosive occurrences and other observations.
Figure 5-1 The reaction between sodium and water is explosive.
Chemical reactions are always happening and are all around us. One does not need
to wear special gear to note their occurrence. For instance the process of
photosynthesis is a chemical reaction plants undergo that involves the conversion of carbon dioxide and water in the presence of sunlight energy to form the sugar
(glucose) and oxygen.
117
6CO2(g) + 6H2O(l) → C6H12O6(s) + 6O2(g)
We would now talk about the different types of reactions known to scientists.
Combination (or synthesis) Reactions
Some reactions involve the coming together of two or more reactants to yield
products. This can be intuitively understood as we know by everyday experiences
that when two or more things combine, better utility could be obtained. For example imagine having a bicycle with amazing design and body work but just a single tire or worse, no tires at all. I really can’t see the point of such a means of transport if you are better off walking. When you add tires however, you find that your bike is more complete and you can enjoy the service it renders. The same goes for combination
reactions. The reactants combine to form products. However, whether the products
are useful or not is largely dependent on their effects.
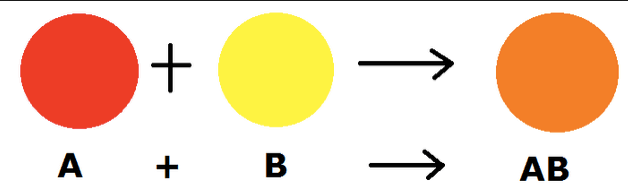
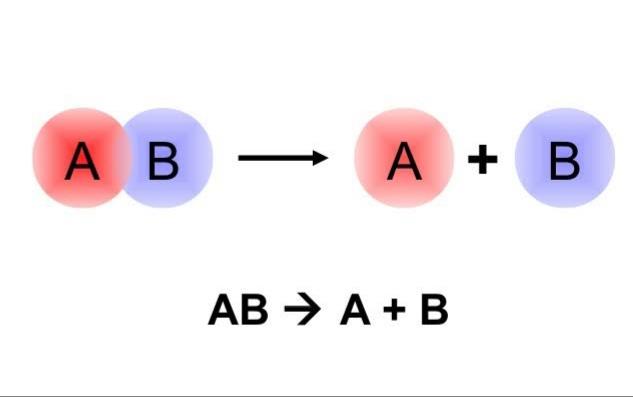
“A combination (or synthesis) reaction is a reaction
that occurs when two or more reactants come
together to form a product.”
Combination reactions of different types can occur depending on what and what
come together to produce something. The general form of a combination reaction is shown below:
A + B → AB
A is the first reactant, B is the second reactant and AB is the product formed when they react.
When two elements come together, they yield compounds.
2H2(g) + O2(g) → 2H2O(l)
Compounds can also equally combine to form other much bigger compounds.
CaO(s) + H2O (l) → Ca(OH)2(aq)
Sometimes an element and a compound may combine.
2CO(g) + O2(g) → 2CO2(g)
118
These are only a few examples of the kinds of combination reactions that occur. We would continue to examine them as we move on in the discussion.
It is important to note that just like me and good sleep, the combination of certain substances just do not work out and reactions never occur with them. An example is one you can do at home. You can try pouring all the oil in the world into a bowl of water but you’ll never get the two constituents to do anything but separate. The
reason why some substances react and others do not would be explored soon.
Decomposition Reactions
While in combination reactions we spoke about adding substances together, in
decomposition reactions we talk of breaking them down. Some compounds can be
reduced into simpler forms. If your awesome bike got a bit old and you being such a crafty wiz decide you could use the individual parts instead, when you take your bike apart you are doing something analogous to a decomposition reaction.
“A decomposition reaction involves the breakdown
of a compound into two or more simpler forms.”
The general form of a decomposition reaction is shown below.
AB → A + B
119
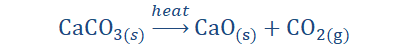
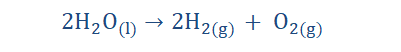
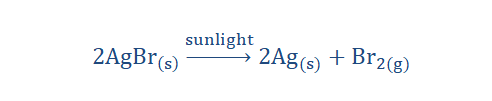
Decomposition reactions are prevalent in the industrial sector. For instance they are used in car airbags; you know the puffy bag that shoots out from the steering wheel when you or someone else is being a little too naughty or is a victim of an
unfortunate collision. The bags emerge due to the breakdown of a compound known
as sodium azide [(NaN3)2] that on decomposing due to an impact produces Sodium
(Na) and Nitrogen gas (N2) which make the bag puff up like that.
When some sources mention decomposition reactions they tend to present them
as thermal decomposition reactions. That is however just one type of
decomposition. You are probably wondering why there are so many ‘types of
types of types’ but things can occur in more than one way and decomposition is
just one of those things. The three types of decomposition reactions are:
1. Thermal decomposition reaction: This type of decomposition involves the application of heat. When the substance is heated it breaks down, forming
simpler substances. It is also termed thermolysis.
2. Electrolytic decomposition reaction: Here, an electric current is introduced and as it passes through an aqueous solution of a compound it breaks it
down and forms simpler elements. Water can be decomposed in this way to
form hydrogen and oxygen.
3. Photolytic decomposition reaction: In this reaction, light is the weapon of decomposition here. More complex substances or compounds on exposure to
light are broken down to form simpler elements. The breakdown of silver
bromide molecules (AgBr) when exposed to sunlight is a perfect example of a
photolytic decomposition reaction. Here the decomposition is observed on
account of a change in color of the AgBr crystals from light yellow to a grayish
color.
Catalytic Reactions
When I was in secondary school I had a classmate who I would call…Anno Yihin
(just a coincidence that it sounds like ‘annoying’). Anno Yihin was disliked by
120
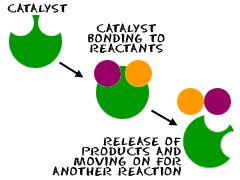
most people because he had the habit of starting fights between good friends
and then slyly disappearing from the scene so that nobody thought to mention
him when an attempt was made to settle the quarrel. He also never seemed to
be bothered by his actions. He could cause fights but was himself unaffected by the situation. Catalysts are just like Anno except their intrusions may be welcome sometimes. They can affect chemical reactions by making them go fast or slow
but are themselves not involved in the reaction. A catalytic reaction is therefore a reaction that includes catalysts to aid product formation. It is also termed
catalysis.
“A catalytic reaction is one that involves the use
of catalysts to aid in the formation of products.”
“Catalysts are substances that alter the rates of
chemical reactions but are themselves
chemically and quantitatively unchanged when
the reactions conclude.”
Just like Anno, catalysts can either speed up or slow down reactions but are
more or less unaffected by the reaction. Their chemical and quantitative
compositions do not alter.
Figure 5-2 Catalysts remain unchanged at the end of a reaction.
Catalytic reactions are of two types depending on the states of the participating substances.
121
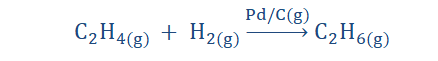
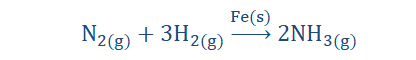
1. Homogeneous catalytic reaction:
In this reaction type all the participating substances are of the same state
as the catalyst. What this means is that each reactant, product and the
catalyst must be liquid, gas or solid.
The reaction shown above is the hydrogenation of ethene and as you may
observe, all participating substances are in the gaseous form. Don’t worry
your head about the process of hydrogenation yet as we would soon get
to that. For now, be content with understanding the concept of
homogenous catalysis.
2. Heterogeneous catalytic reaction:
Catalysis of this nature is the opposite of homogeneous catalysis. Here,
the different participating substances are in different phases from the
phase of the catalyst.
While catalytic reactions are of two types as seen above, catalysts are
also of a number of types. Some catalysts occur naturally and are hence
called organic catalysts while those synthesized in the laboratory are
inorganic catalysts.
1. Organic catalysts:
These catalysts are involved in organocatalysis which is just a type of
catalysis consisting of an organic catalyst. They typically consist of
carbon, sulfur and hydrogen-elements found in organic compounds. A well
known example of an organic catalyst is an enzyme. Enzymes are
biological catalysts that affect the rates of reactions that occur in the
bodies of organisms.
2. Inorganic catalysts:
These catalysts are usually synthesized in the laboratory as opposed to
being made in the bodies of living creatures. They are divided into two
types; positive and negative catalysts.
122
Positive catalysts are inorganic catalysts that help to speed up the rate at which a chemical reaction is going. Good examples of positive catalysts
are potassium permanganate (KMnO4) and Manganese dioxide (MnO2).
These catalysts are used in a host of industrial processes such as in the
making of pesticides, air treatment and automotive care.
Negative catalysts on the other hand retard the rates of chemical
reactions. Examples are phosphoric acid (H3PO4) and Tetra Ethyl Lead
(T.E.L).
It is important to know that catalysts themselves can be affected by the addition of another substance. They can be made to be more efficient or have their
tendency for speed dampened. Substances that improve the efficiency of a
catalyst by making it speed up a reaction even more are known as a promoters
or accelerators. Those that do just the opposite, reducing the efficiency of a catalyst are inhibitors.
When I learnt about catalysis for the first time, I couldn’t wrap my head around
why promoters and inhibitors weren’t catalysts themselves. It just made a whole
lot of sense that they could be called catalysts not just because I wanted to
memorize fewer names but because they seemed to do the same thing catalysts
do. Took me a while to realize that while catalysts are concerned with the
reaction itself, promoters and inhibitors are concerned with the catalysts and so they have little to no bearing or effect on the reaction.
Thermal dissociation reaction
I can see into the near future. I got that ability from staying awake so long that things got a little strange. My ‘that’s so Raven’ inspired senses are telling me that you may get a little confused in a while. But don’t worry. We’ll get through it.
Not long ago we spoke of thermal decomposition. We defined it as the
breakdown of a compound or larger entity into simpler substances on account of
the application of heat. Now we would be talking of another process similar to the breaking down of a compound due to heat but different in a few ways.
123
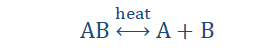
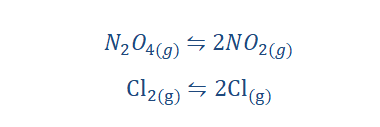
“Thermal dissociation reaction is a reaction that
involves the splitting of a compound or more
complex entity into its simpler compositions.”
Yes, I know. It does sound like a lot like decomposition but it really isn’t.
Picture this. Imagine I give you and Anno Yihin (yes, he’s back) sheets of paper
that are my very important credentials and ask both of you to ruin them in such a way that I could fix them again. You decide to only tear the sheets of paper in
half, aware that you could put them back together with some glue. Anno on the
other hand being such a peaceful young man decides he would do the same,
however he doesn’t stop there. He decides to crumple the papers, chew them
and then stomp on them after which he places them in a bowl of water and
makes ‘credential soup’. He undergoes so many stages of destruction as though
he were a positive catalyst. As you can probably see your papers can return to
their initial states with just some glue but his can’t. Your actions with the papers are reversible but for Anno there are irreversible. He can never get the papers back the same way. He can probably also never get his bones back the same
way if someone breaks them.
The same reasoning applies for decomposition and dissociation reactions.
Decomposition reactions are largely irreversible and are mostly naturally
occurring. Dissociation reactions on the other hand are reversible. After the
products are formed, they can revert to the reactants again.
Thermal dissociation reactions are of the form;
As you can see above the arrow points in both directions meaning that the
reaction can go in both ways under the right conditions (right conditions of
temperature and pressure). Some examples of thermal dissociation reactions
are;
124
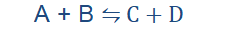
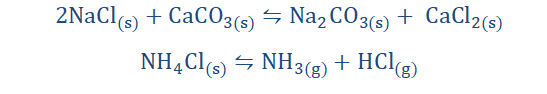
Reversible reactions
Many of the reactions we have considered before now have been irreversible
reactions. They have only proceeded in one way.
A + B → C + D
Reversible reactions occur in both directions. Thermal dissociation is one
instance of a reversible reaction as we have just seen. Reversible reactions must not require heat to occur. All reactions that can be reversed irrespective of the means used to do this are reversible reactions. They are usually of the form;
It is fascinating to note that for as long as possible chemists had believed that all reactions could only proceed in one way. It took some out of the box thinking for Claude Louis Berthollet who proved this wrong in 1803.
Reversible reactions may only occur under certain conditions such as at specific
temperatures and pressures. They are particularly interesting because the
formation of products and the reverse (formation of reactants) can take place
simultaneously when the conditions required both for product and reactant
formation are similar. What this implies is that the process may be ongoing until equilibrium is met. Equilibrum happens when the amount of products formed balances the reactants reformed and the process can then be brought to a stop.
It’s like having Anno and someone you find displeasing engage in exchanging
blows until both of them tire out and maybe get concoctions. Their hits were
reversible until there was balance in the form of their double concoction.
Some examples of reversible reactions are:
Displacement reactions
Just as the name implies, a displacement reaction is one concerned with
replacing some participating entities (atoms, molecules, elements) in a reaction
with other participating entities.
125
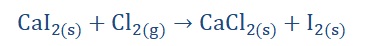
“A displacement reaction is one in which one or
more atoms of an element displace
another/others in a compound.”
Displacement reactions are of the form;
There are of two types. These are single (substitution) displacement reactions and double (metathesis) displacement reactions.
Single (substitution) displacement reaction:
This reaction involves the replacement of only one atom of an element in a
compound by another.
The I2 in CaI2 on the reactant side was replaced by Cl2 leading to the formation of CaCl2 and the displacement of I2. An amusing way to visualize a single
displacement reaction is to imagine that a loved one bought you a new pet. I can
picture you dancing around with your pet puppy all over the place. But then one
day our ‘friend’ Anno shows up at your door and takes the pet forcefully from you because he has something you don’t, maybe special puppy food and you are left
alone, seemingly replaced by Anno. Sounds sad, but it satisfies our aim of
explaining what a single displacement reaction is.
Another way to picture it is to imagine going to a school dance with your friends.
Your friend Tunde comes with his date and is having a good time showing off his
moves when suddenly Anno shows up and is surprisingly a better dancer,
winning Tunde’s date over with his moves. Tunde is left alone. Hopefully he won’t let that stop his dancing. He should also probably find a better date next time.
Figure 5-3
126
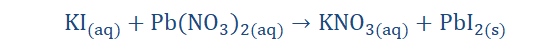
Double (metathesis) displacement reaction:
Here two atoms are being exchanged, forming two new compounds as opposed
to one.
We can see from the equation that the compounds seem to undergo some sort of
exchange. Potassium (K) accepts the nitrate (NO3) from lead (Pb) and lead (Pb)
receives iodine (I) from Potassium (K).
Let’s imagine that a friend of yours Chika sees what happened to Tunde and
decides to dance with him. At that moment the teachers chaperoning the dance
decide to be involved because it seems the ‘cool’ thing to do (gulp). As Tunde
dances along and gets into the music he switches partners and notices that he
now dances with his biology teacher Mrs. Biohr Logia (awkward) while the
Economics teacher Mr. Ecoo Nomica gives Chika a twirl. Well, things seem to be
getting out of hand but the important thing here is that a double displacement has taken place. Tunde and Chika switched dance partners with Mr. Ecoo Nomica
and Mrs. Biohr Logica on the dance floor. You could also join in on the dance
floor and see what reactions you may partake in.
Figure 5-4
It is easier to think of single displacement reactions in terms of a replacement while double displacement reactions seem more of an exchange.
Combustion reaction
Any reaction that involves the evolution of heat and typically involves burning in oxygen is a combustion reaction. Oxidants are substances that can induce
combustion in other substances. Oxygen is regarded as a significant oxidant but
other substances such as fluorine are oxidants as well. The release of heat that
occurs in a combustion reaction is usually accompanied by light. A combustible 127
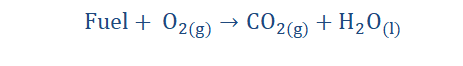
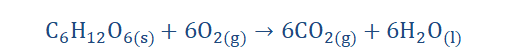

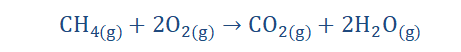
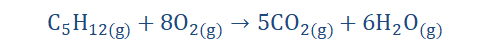
substance that is capable of burning in an oxidant as is composed of combustible
substances is known as a fuel. These reactions have the form:
Combustion reactions are very common. If you light a candle, burn some firewood or anything that burns you have witnessed a combustion reaction. My most memorable
experiences when going to the village for Christmas always involved the smell of
burning firewood in the cold mornings. I loved to experience this because I could tell the chemistry of the process and that was thrilling. The reaction below is an equation roughly showing the combustion of wood (cellulose) as it is a multi-step process in reality.
Figure 5-5 Combustion reactions are not always as adorable as small Christmas time fires.
The smoke we observe when we burn something is usually a gas mixture that is the
product of the reaction. Smoke produced from incomplete combustion tends to
contain unburned particles of the fuel; that we see however when a complete
combustion reaction takes place, H2O and CO2 become the only products. Some
examples of combustion reactions are shown below;
In the above we see the combustion of methane, a hydrocarbon.
Here we have the combustion of pentane which is another hydrocarbon. No, I’m not
speaking Spanish (I wish), it’s just chemistry. We’ll find out about hydrocarbons in time.
128

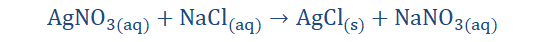
Precipitation reaction
Figure 5-6 Precipitation reaction
Every time I insist you get a drink before continuing our little discussion I get one for myself as well. I like the taste of lemons so I get a sachet of lemon tea. I really enjoy drinking it but it has one problem; in hot water the contents of the sweet lemon tea dissolve easily, but when I put it in cold water they don’t. They just float around in clumps. This is quite frustrating especially since I enjoy and need cold drinks to cool my head down after all this chemistry. Precipitation reactions are similar to my cup of cold lemon tea. When reactants react, they form products and one of those products is usually insoluble in solution and would just float around or precipitate out of the solution. The liquid left behind in the formation of a precipitate is a supernate.
“A precipitation reaction is defined as one that
involves the reaction between two soluble
reactants in an aqueous solution to form products;
one of which is insoluble.”
Precipitations can also be defined in terms of the reaction between cations and
anions in aqueous solutions to yield ionic solids that are insoluble. An example of a precipitation reaction is shown below;
If you are keen- and I can tell you are- You would probably be saying something now in the lines of; “Hey! That’s double displacement!” and of course I have some
explaining to do.
Just like thermal dissociation was a kind of reversible reaction, precipitation
reactions can be single and double displacement reactions. The only catch here is 129
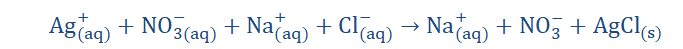
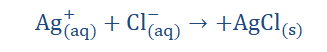
that for double displacement reactions to be precipitation reactions there must be the formation of a solid, insoluble product and an aqueous solution. All these conditions are fulfilled by the reaction shown above. Single displacement reactions also
become precipitation reactions when one reactant ion is being replaced by another and a new solid product is formed.
It can be difficult to understand these reactions from an ionic perspective. Let’s clear up that confusion before moving along.
The above is the ionic equation of the reaction stated above. What we are presently doing is trying to arrive at the net ionic equation of the reaction. The first thing we have done here is separate the individual ions of the reaction. I am certain you recall what ions are. If you do you would realize that the elements with the superscript ‘+’
are cations; having one to a few electrons on their valence shells to give away.
Those with the ‘- ‘superscript are anions and so have to receive electrons to
complete their octet shells. When these exchanges take place, the aqueous and
solid products are formed. When we cancel out the ions repeated on both sides of
the equation (spectator ions) we get;
After this I am quite sure the definition of precipitation at the start of this section is now clearer to you. Now imagine if all the participating entities were aqueous
meaning that none would be solid. Would you still be able to obtain a precipitate? I’ll wait.
Solid-state reaction
Be honest. Before you began studying chemistry and became a bonafide chemist
yourself, when you pictured chemists working you always imagined them holding a
test tube with some kind of liquid in it, looking studiously at the content of that test tube. Can’t count how many Chemistry textbook covers look like that. We seem to
always talk about liquids in chemistry that the only times we refer to solids is when we have to do something to them with liquids. This is understandable as liquid
molecules are freer and hence much more available to react than solid molecules.
Nonetheless reactions between just solids also take place and those reactions are known as solid-state reactions or solid state synthesis.
130
“Solid state reactions are reactions between solid
substances to form products.”
Due to the fact that solid substances have a more rigid structure, it is usually
necessary to grind the reactants in order to allow for proper mixing and hence a
reaction to occur. Some solid state reactions may also only occur under specific
conditions such as at very high temperatures. Surface area and reactivity are also factors taken into account when carrying out solid synthesis.
Reduction and oxidation reactions (REDOX)
Chemical reactions can be analogous to passing in a game of basketball. When one
player passes the ball, the other player receives it. It’ll make no sense for the player catching to intentionally miss the ball, unless he was just a bad basketball player, like I was in my university team. The same thing happens in a reduction and
oxidation reaction. One of the reactants gives off an electron that is received by the other reactant. The reaction is therefore divided into two halves; the first is the oxidation reaction and the second is the reduction reaction.
Oxidation is therefore defined as the loss of electrons, while reduction is defined as the gain of electrons. Saying ‘reduction and oxidation’ takes a lot so we would call it a redox reaction instead.
“An oxidation-reduction reaction (redox) reaction is
defined as the transfer of electrons between
reactants to form products.”
“Oxidation is defined as the loss of electrons
during a chemical reaction.”
“Reduction is defined as the gain of electrons
during a chemical reaction.”
131
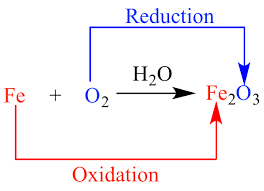
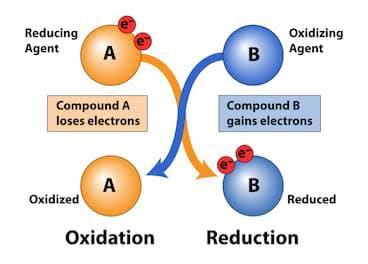
The reactant that is giving its electron(s) away is known as the reducing agent while the reactant accepting the electron(s) is the oxidizing agent. Don’t look confused, it takes me back to when I learnt it. I remember how easy it was to mix up the two. If oxidation is the loss of electrons then why is a reducing agent losing the electrons?
Let’s clear this up.
Figure 5-7 Explaining the redox reaction
In the above we find the reaction between iron (Fe) and oxygen (O2) to form
iron(II)oxide. Iron loses electrons and oxygen gains them. Since Fe is losing the electrons it is becoming oxidized. O2 is gaining the electrons and so is being
reduced. Since Fe is being oxidized, it is reducing O2 so it is the reducing agent. O2
on the other hand is accepting electrons and so is undergoing reduction but at the same time it is oxidizing Fe and so it is the oxidizing agent.
Figure 5-8
Oxidation states
An element’s tendency to lose or gain electrons can be described by considering the oxidation state of that element.
“The oxidation state of an element shows the
number of electrons of an atom of the element that
132
is involved in bond formation with the atom of
another element.”
The oxidation state of an element can sometimes be referred to as its oxidation
number.
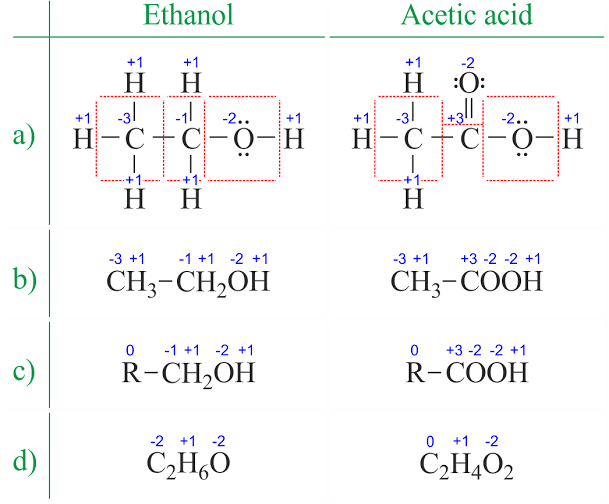
Figure 5-9
The above image shows the different ways by which the oxidation states of atoms of elements can be deciphered. Here we have ethanol and acetic acid. In (a) let’s find the oxidation state of the first carbon (C). As you can see this C is bonded to 3 H
and one other C. Each H contributes one electron to the bond and C contributes one each. For the H-C bonds, we have 6 electrons in total (remember that 1 ‘dash’
signifies 2 electrons). So the first C gains 6 electrons from these bonds. However when we talk about the C-C bond, we do not include 2 electrons but consider just 1
electron. This is because for bonds between atoms of the same type, we divide them equally. The oxidation state of the first C is then gotten by subtracting the bonds (number of electrons) that the C has gained from the number of valence electrons in a C atom. For some elements we would also consider lone pairs of electrons
(electrons that do not form bonds but stand alone) but that does not apply in this case as all four valence electrons of C are involved in bond formation. We can use an equation to represent this;
133
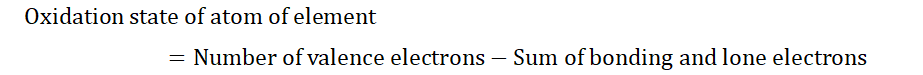
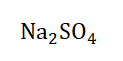
We may not always be able to spread up a compound like that to notice what bonds
with what and so in order to know the oxidation state of an element relatively easily we follow a set of arbitrary rules. These rules for assigning oxidation numbers are;
1) The oxidation states of free elements such as O2, N2 and so on are assigned an oxidation number of 0. Free states are when elements are stable and
largely unreactive in that state. O2 for instance is diatomic in the free state.
2) The oxidation number of H2 is +1 unless it is found in compounds that have
elements less electronegative than it is. In that case it becomes -1.
3) Oxygen usually has an oxidation number of -2. This could change however on
account of the extent of electronegativity of the elements it can be found with
in a compound.
4) In writing a formula, the cation should be written first before the anion. An interesting example can be found in the compounds NaH and HCl. The
oxidation number of H in HCl is +1 and so being the cation it is written first.
However in NaH, the oxidation number of H is -1 and so H being anionic
comes after Na.
5) The charge on an ion is not the same thing as its oxidation number. Though
in the case of monoatomic ions this is so. The oxidation state of Na for
example is +1 (Na+).
6) The oxidation number of a neutral compound is 0, implying that the sum of
the oxidation numbers of the atoms in a neutral compound is 0.
7) For polyatomic ions, the oxidation numbers equal the charges on the ion. For
instance for NO -1
3 the oxidation number is -1.
The concept of oxidation numbers can be a difficult one to wrap your head around.
In fact since the beginning of this section, redox reactions have given us nothing but trouble. But just take a deep breath and read through it a few more times and it
would become clearer to understand. And of course, I’ll help. Have a look at the
compound below;
134
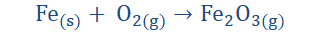
Imagine you are asked to give the oxidation number of S in the formula how would
you go about it? I’m quite sure you suggested that we follow the oxidation numbering rules. Let’s do just that.
First we note the oxidation state of Na which is +1 in accordance with number 5 of the rules; as it is a monoatomic ion with a charge of +1 (remember?). As for oxygen its oxidation number is -2 following number 3 of our ‘oxidation number’ seven
commandments. So it seems the only oxidation number that eludes us is the one for sulfur (S) but that isn’t a problem. Recall that he oxidation number of a neutral compound is 0 and Na2SO4 is a neutral compound. Now all we have to do is try to
make the resultant oxidation number 0.
If Na is +1 and O is -2 and we have 2 Na atoms and 4 O atoms, we simply each
charge by the number of electrons and then add them together to obtain 0.
Na2SO4 = 0
(+1 × 2) + S + (-2 × 4) = 0
2 + S + (-8) = 0
2 + S – 8 = 0
S = 0 + 8 -2
S = +6
Let’s see if this is accurate by adding all the charges and seeing if it equals 0.
Na2SO4 = 0
(+1 × 2) + (+6) + (-2 × 4) = 0
2 + 6 + (-8) = 0
2 + 6 - 8 = 0
8 – 8 = 0
Since Na2SO4 is a neutral compound and the oxidation numbers add up to 0, we
know for certain that the oxidation number of S in the compound is 0.
Understanding redox reactions in the scope of oxidation numbers
Now that we are fairly certain we get the gist of oxidation numbers, we can go on to applying the idea to redox reactions. Have a look at the equation below.
Since oxidation is the loss of electrons and reduction the gain of those electrons, one of these species is losing electrons and the other gaining. We have already
established that the reducing agent is giving off electrons while the oxidizing agent is 135
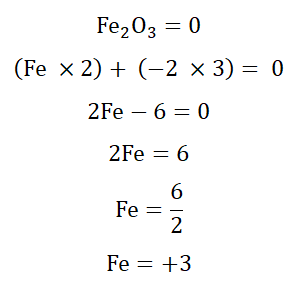
accepting the electrons. Geared with this knowledge and the oxidation assignment
rules, we can now learn how their oxidation numbers change.
Both Fe and O have oxidation states of 0 at the start of the reaction as there are both in the neutral states in accordance with the first law. On reacting however, their oxidation numbers change. We have already learnt that the oxidation state of O in a compound is -2. We should also be aware that Fe2O3 is a neutral compound.
If you input this value in the formula and add them together you would find that it equates 0, showing that it is correct.
So now we see from the equation that the oxidation state of oxygen changed from 0
to -2 while the oxidation state of Iron changed from 0 to +3. O gained electrons and went from being neutral to being a negative ion (anion) while Fe lost electrons going from a neutral atom to a positive ion (cation).
The loss of electrons or oxidation took place when the oxidation state of Fe changed from 0 to +3 while the gain of electrons (reduction) happened when O changed
oxidation state from 0 to -2. Since Fe was making O accept electrons it reduced O
and so is the reducing agent and since O made Fe give up its electrons it was
oxidizing Fe and so is the oxidizing agent. This new information grants us an
alternative definition of a redox reaction.
“An oxidation-reduction (redox) reaction is one in
which the oxidation number of a specie (atom or
molecule) alters by the loss or gain of an electron.”
136

“Oxidation is defined as the increase in oxidation
number of a reacting specie during a chemical
reaction.”
“Reduction is defined as the decrease in oxidation
number of a reacting specie during a chemical
reaction.”
Figure 5-10 The rusting of iron is a common example of a redox reaction. Fe reacts with O2
to form Fe2O3.
Neutralization reaction
When a substance known as an acid reacts with another substance known as a
base they form the products salt and water. Such a reaction is a neutralization
reaction.
Acids
“An acid is a substance that donates protons
(hydrogen ions) on dissolution in water.”
This is only just one definition of an acid and it is attributed to Johannes Nicolaus Bronsted and Thomas Martin Lowry. It is therefore appropriately named the
Bronsted-Lowry acid. They proposed this definition in 1923. Acids have undergone 137
a number of definitions and are so named depending on the definition. Svante Arrhenius defined an acid as;
“…Any substance that increases the hydronium
(or oxonium) ion (H3O+) concentration in solution.”
And so it is named the Arrhenius acid. The hydronium ions referred to here are simply just the outcome of H+ (hydrogen ions) dissociating from the acid to form
bonds with the water. Lastly;
“An acid is a substance that accepts electron
pairs.”
This was proposed by Gilbert N. Lewis (remember him?) and is the Lewis acid.
Acids can be strong acids or weak acids.
Strong acid: When an acid ionizes or dissociates completely in solution, it is known as a strong acid. Examples of strong acids are HCl, HBr, HI, H2SO4, HNO3, HClO4
and HClO3. There are only about a handful of substances that ionize completely in solution producing hydrogen ions and the seven mentioned here are the only strong acids known which to me is pretty mind blowing. All the other acids besides these you may come across are very likely weak acids.
Weak acids: These are not completely ionized in solution. They dissociate only partially and so can co exist in solution with their conjugate bases. Conjugate bases are acids that lose hydrogen ions which are accepted by bases. If the base is a
weak base and does not ionize completely it would only partially receive hydrogen ions and hence form a conjugate acid. This would be explained briefly.
Acid characteristics
Though the underlying basics for the behavior of an acid is the existence of H+ in solution, acids can be identified on account of the properties that they possess.
These properties are listed below.
1) They turn blue litmus red: Litmus paper is a special kind of paper; special
because it has undergone treatment with particular dyes to change color
when placed in contact with an acid. When blue litmus paper is dipped in
acid, it turns red.
138
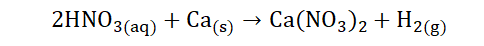
2) They are corrosive and just downright dangerous when concentrated.
3) They have sour tastes: Am almost certain you have tasted an unripe fruit
before. Believe it or not you actually tasted a weak acid (usually acetic acid).
4) They have a PH of less than 7: PH is a scale used by chemists to determine
whether something is acidic or basic. You would soon come to understand
this fully after we talk about bases.
5) They react with bases to form salt and water (neutralization).
6) They generally react with metals to liberate hydrogen gas: This is noted below when nitric acid (HNO3) reacts with calcium (Ca) to form the salt calcium
nitrate [Ca(NO3)2] with H2 liberated.
Acids can be classified into organic acids and inorganic (mineral) acids. The organic acids occur naturally (such as the taste of unripe fruits) while the mineral acids are synthesized in the laboratory.
Bases
You have already seen that bases are the substances that react with acids to
form salt and water. They can also be said to be the substances that produce
hydroxide ions in solution.
“A base is a substance that increases the
hydroxide ion (OH-) concentration in water.”
So while acids increase the concentration of hydronium (H3O+) ions in water,
bases increase hydroxide ions (OH-). Both ions combined produce a water
molecule.
Just like acids bases can be termed Arrhenius, Bronsted-Lowry and Lewis bases with corresponding definitions.
Base characteristics
Just like acids, bases have some specific characteristics. These are;
1) They turn red litmus paper blue.
139
2) They have a slippery texture.
3) They have a characteristic bitter taste.
4) They have a PH of above 7.
5) They react with acids to form salt and water.
6) They can be either soluble or insoluble in water. Soluble bases are known
as alkalis.
Bases are divided into strong bases, weak bases, super bases and solid bases.
Strong bases: These are bases that completely ionize in water to produce
hydroxide ions (OH-). There are also known as substances that remove H+ with
ease from weak acids. Remember that H+ ions are what make substances acidic;
when dissolved in water they dissociate to produce those hydrogen ions.
However in water the H+ become H3O+. Bases that can remove those hydrogen
ions from acids are strong bases and this process is termed the basicity of an acid. Note that some acids are more basicity than others, meaning they have more replaceable hydrogen ions in their molecules.
“The basicity of an acid shows how many
replaceable hydrogen ions there are in one
molecule of the acid.”
How many H+ that can be removed by strong bases can be known from just
looking at the formula of the acid. For instance sulphuric acid (H2SO4) is dibasic since we see two hydrogen ions that can be removed by a strong base (2H+,
SO 2-
4 ). For nitric acid (HNO3) the basicity is 1 and so the acid is monobasic; as
one hydrogen ion can be removed by a strong base (H+, NO -
3 ). What do you
think would be the basicity for phosphoric acid (H3PO4)? I’ll wait. An example of a strong base is potassium hydroxide (KOH).
Weak bases: Weak bases only ionize partially in water to produce hydroxide ions and so in solution they tend to exist with their conjugate acids. Conjugate acids are formed when a base accepts hydrogen ions from an acid. So a
conjugate acid is basically a base with a hydrogen ion added to it. They are only possible when incomplete ionization occurs as in the case of a complete
dissociation of a base into OH- a strong base would occur. To make this easier to understand, note that the weaker the conjugate acid present, the stronger the
140
base and the weaker the base, the stronger the conjugate acid. One example of
a weak base is ammonium hydroxide (NH4OH).
Super bases: These are strong bases with capes. They are even better at
dissociating completely to form bases and removing H+ from acids.
Orthodiethynylbenzenedianion (C6H4(C2)2)2-) is the strongest superbase known. I
hope you didn’t hurt yourself saying that.
Solid bases: Any base in solid form can be considered a solid base. An example is sodium hydroxide (NaOH).
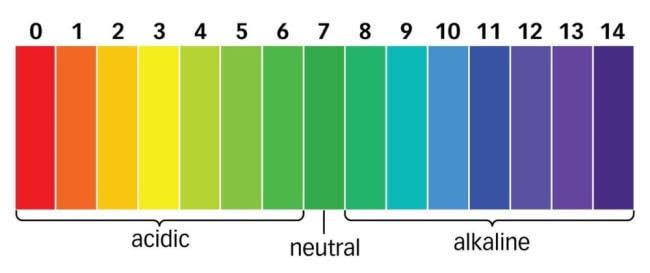
The PH Scale
Figure 5-11
Some very special dyes show color changes when placed in contact with basic,
acidic or neutral substances. These dyes are varied and the litmus paper we
talked about earlier is just one of them. Litmus papers are a treated dye obtained from lichens which is soluble in water. The color change is based on the
concentration of ions in any given solution. Red litmus papers turn blue on
exposure to alkaline or basic substances while blue litmus papers turn red when
placed in contact with acidic substances. Neutral litmus papers could be either
blue or red on account of how basic or acidic something is.
The depth of the color shows the extent of the acidity or alkalinity of something.
As seen above in the image, it is a scale. A value of 1 shows that the substance
is strongly acidic with a deeply red color to indicate that. Neutral substances are green and alkaline substances go from blue to deeper shades of purple. This
variation is the PH scale.
“The PH scale is a graduated measure of how
acidic or alkaline a substance is.”
141
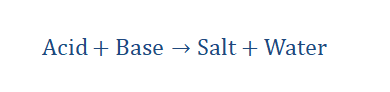
Litmus paper is just one indicator of this scale. Other indicators used are
phenolphthalein, bromothymol blue and methyl orange. You would use some of
these in laboratory work.
Neutralization
Here at last. We needed to understand acids, bases and PH to explain
neutralization reactions. Now you know the properties of acids and bases and it
was all nice knowing them but in neutralization reactions all those properties in a sense, become destroyed. When acids and alkali react, they form substances
that differ greatly from them and their combining qualities. They form salt and
water.
How they differ you ask? In order to answer that we’ll have to note the properties of salts and so understand what they are.
Salts are formed when acids and bases react. They are usually powdered in
form. They are of two types; acid salts and basic salts. Acid salts are salts that produce acidic solutions, while basic salts on dissolution in water produce
hydroxide ions and hence basic or alkali solutions. You may be wondering where
our common everyday table salt fits in all this. Table salt is NaCl and is a neutral salt. These salts are neither acidic nor basic and thank God for that because if NaCl were either of those we would suffer from mostly tasteless food.
Salts have many colours, from transparent (as in sodium chloride) to violet (as in potassium permanganate). They also come in a variety of tastes from salty
(sodium chloride; and funny how the ‘salt’ is ‘salty’) to bitter (magnesium sulfate) and even sweet (lead diacetate). It may be tempting to think lead diacetate candy is a good idea but that sweetness may be the last thing you ever taste; so no.
Other salts can be savory or umami. They show odor variety as well as varying
solubility.
The point made here is that salts have a plethora of variations that differ from the properties shown by their parent acids and bases. The following are examples of
neutralization reactions.
142
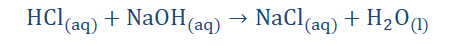
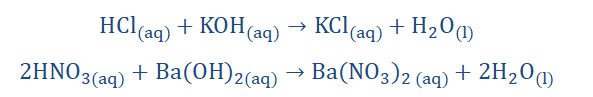
An everyday example of a neutralization reaction is when you take antacid
tablets or solutions when suffering from stomach acidity. The antacid solution is alkaline as you can probably notice on account of it being quite slimy. When you
take it, it neutralizes the acid in your stomach and returns the PH of your
stomach to a neutral state. So thank neutralization when you feel better after
taking a spoon of Gestid next time.
We’ve been talking so long that you definitely need a drink. I may also need one of those antacids. Why not take a while off to rest? When you’re back we would
be trying to understand exactly how and why any of these reactions happen; we
would be discussing chemical kinetics.
143
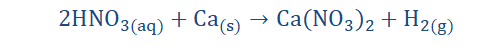
Chemical Kinetics
We have already defined chemical kinetics as the study of the rates of reactions.
In kinetics we attempt to know how fast or slow reactions go and the underlying
reasons for those rates. We have also defined a reaction rate as the time taken
for a reaction to reach completion. For instance;
The rate of this reaction can be obtained by noting how fast the Ca is used up by the acid, and how fast the products are formed. So reaction rate can be observed
by noticing how the masses of reactants decrease or are used up and
products are formed.
Another way to note the rate of a reaction is to measure the changes in
concentration of the participating entities as the reaction proceeds. In the example above, we can measure the decrease of concentration of the reactants
and subsequent increase in product concentration.
Sometimes the volumes of reactants alter on account of a reaction taking place.
When we measure volume changes of reactants in the formation of products,
we can measure rates of reactions.
In precipitation reactions we could measure how fast or slow a reaction is by
measuring how much precipitate is formed at the end of the reaction.
Some reactions rates can be measured when the reactants change color as the
products are formed. The speed at which the color changes occur correlates to how fast the reaction is going.
When the PH of a reaction changes, measuring the rate of that change could show if the reaction is fast or slow.
The collision theory
I am not the biggest fan of Mixed Martial Arts (MMA) but anytime I happen to
watch a fight I stay at the edge of my seat because the matches are just so
thrilling. Here I find men and women throw punches and kicks at each other but
remain standing, ready to punch and kick again until one of them takes a bad
enough hit and reacts to it by falling.
144

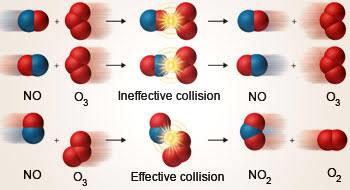
Figure 5-12 MMA fighters…make me feel the need to work out more
What happens in these fights is somewhat analogous to what happens when
reactant particles give rise to products. The particles of the reactants constantly collide with each other however just like not all hits in a fight can cause a knock out, not all collisions between reactant particles lead to products. Those that do lead to the formation of products are termed effective collisions. Effective collisions are not as many as non-effective ones. The non-effective collisions
simply result in the rebounding of the particles the same way a ball bounces off a wall. Imagine if every hit given and received by MMA fighters were effective
enough to cause knockouts. I don’t think the fights would be nearly as interesting.
But what happens when the reacting particles collide? The thing is that the
particles on collision break their existing bonds so new bonds can be formed. A
visual aid may be more effective in explaining this.
Figure 5-13 Illustrating effective collisions
As you can see from the image the ineffective collisions do not result in product formation but the particles reacting simply bounce off each other. Effective
145
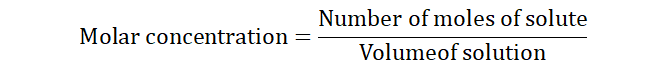
collisions result in bonds breaking and new bonds being formed thereby forming
products.
This is all nice and dandy but there’s a catch. Before reactant particles can break bonds, they must have sufficient energy to do so. This energy that they are
required to have to initiate bond breaking is called the activation energy.
Imagine if one of the MMA fighters mentioned above had indulged in eating
unhealthy and watching TV frequently prior to the match. She would never have
had enough energy to knock out her opponent who may have been working out a
lot during that time. So in the same way reactant particles must possess enough
energy to break bonds and form products. If the reactant particles have energies
lower than the activation energy, the reaction would not occur but if their energies exceed the activation energy, it would.
The lowering of this activation energy is what makes catalysts so good at
speeding up the rates of reactions. When added, a catalyst lowers the activation
energy, making it easier for reactant particles to form products and hence do it
much faster. Negative catalysts do just the opposite by raising the activation
energy instead.
Practical ways to make reactant particles exceed this energy barrier may be to
heat up the reacting system or introduce an electric current.
Factors that influence reaction rates
Some factors can affect how fast or slow a reaction may be. So far we have seen
some of these factors. We have seen catalysts, have considered particle collision rates and have noted the energy the reactant particles must possess to induce a
reaction. But apart from these there are many other factors that could affect the rate of a reaction. These are listed below.
1) Concentration of reactants: Concentration of a substance is defined as
how much of the substance (amount of a substance) is available in a
given volume of solution. It is also known as the molarity of a substance
and is measured in molsdm-3.
When there is a high concentration of reactants present in a solution,
chances are that the frequency of collisions would be correspondingly high
146
meaning that effective collisions would also increase. This of course means that more of the products would be formed. The reverse of this
(low reactant concentrations) would mean that less amount of product
formation would occur.
2) State of reactants/surface area of reactant particles: Remember when we
spoke of solid state synthesis? We mentioned that the only way to have
the solids react well was to grind them into powdered form so as to
increase the area of contact between them. So increasing how much area
of the reactant particles are in contact with each other would ensure that
greater collisions take place. Imagine having to cause a reaction between
substances of different states; maybe a liquid and a solid. If you do not
grind the solid to increase its surface area and just dump it in there,
chances are you would get very little reactant collisions because not much
surface area is there to ease the reaction process.
3) Nature of reactants: Some solvents react much more easily with solutes or other substances than some others. This has nothing to do with the
mechanism of reaction itself, but has much more to do with the inherent
properties of the solvent. A piece of Na may not react when dropped in
kerosene or some other inert liquid, but on slight contact with water we get
an explosive reaction (check the first image of this chapter to see this).
This does not only apply to solvents. When the same piece of Na is placed
in N2 gas it seldom reacts. It reacts with hydrogen however, forming
sodium hydride (NaH). A recent study has even shown that sodium reacts
with the inert gas He, though this only occurs under very special
conditions where He gas is placed under a high enough pressure. So we
see that the nature of the reactants affects the reaction rate.
4) Temperature: If you raise the temperature of a reaction system, you
increase the energy acquired by the reacting particles. As we have
already learnt before now, when these particles acquire this energy they
move about much more frequently and with much speed colliding with one
another. This therefore means that the chances of obtaining effective
collisions rise and so the reaction rate proceeds much faster. Below is a
graph showing the relationship between the temperature (in K) and rate of
a reaction to prove this.
147
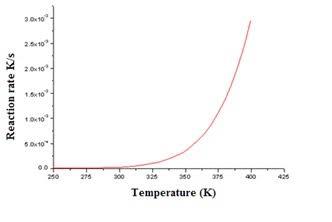
Figure 5-14 Graph of Temperature against Reaction rate
5) Catalyst effect: Catalysts can speed the rate of a reaction by lowering the
activation energy of the reaction system allowing the reactant particles
overcome the energy barrier. In order to slow down the rate of a reaction
they do this by increasing the activation energy, making it difficult for the
reactant particles to get pass this energy barrier. One amusing way to
picture the effects of catalyst is to imagine playing basketball with a small
child. You know that asking the child to score some baskets is virtually
impossible, so in order to ensure that the game is over quickly you lower
the hoop every time he tries to shoot because you know that if left to him,
you may never leave that place. You play the part of a positive catalyst,
the hoop is the activation energy, the child is the reactant particle and
making the basket is the reaction. Let’s say Anno is now playing
basketball with the same child. Being such a kind young man, whenever it
is time for the child to score he raises the hoop even higher. He’s a
negative catalyst and not even the child’s tears could affect him.
And there we have it. Now you know the factors that affect the rates of chemical
reactions. Let’s move on to answering some questions and see how much you’ve
really learnt.
148
SUMMARY
Chemical kinetics is a branch of chemistry that deals with the study of the rates of chemical reactions.
A combination (or synthesis) reaction is a reaction that occurs when two or more reactants come together to form a product. The general form of a combination
reaction is A + B → AB.
A decomposition reaction involves the breakdown of a compound into two or
more simpler forms. The general form of a decomposition reaction is AB →A+B.
The three types of decomposition reactions are thermal decomposition,
electrolytic decomposition and photolytic decomposition reactions.
A catalytic reaction is one that involves the use of catalysts to aid in the formation of products.
Catalysts are substances that alter the rates of chemical reactions but are
themselves chemically and quantitatively unchanged when the reactions
conclude.
Catalysis is divided into homogenous and heterogeneous catalysis.
Catalysts can be organic or inorganic catalysts. Inorganic catalysts are largely positive or negative.
Thermal dissociation reaction is a reaction that involves the splitting of a
compound or more complex entity into its simpler compositions.
Thermal dissociation reactions are mostly reversible, thermal decomposition
reactions are mostly irreversible and largely naturally occurring.
Reversible reactions are reactions that can proceed in both directions.
A displacement reaction is one in which one or more atoms of an element
displace another/others in a compound.
There are two types of displacement reactions: single (substitution) and double (metathesis) displacement reactions.
Any reaction that involves the evolution of heat and typically involves burning in oxygen is a combustion reaction.
A precipitation reaction is defined as one that involves the reaction between two soluble reactants in an aqueous solution to form products; one of which is
insoluble.
Precipitation reactions can be single or double displacement reactions.
149
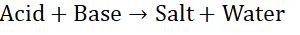
Solid state reactions are reactions between solid substances to form products.
Reduction and oxidation reactions are to halves of a single process.
A redox reaction involves the transfer of electrons between reacting species,
oxidation is the loss of electrons while reduction is the gain of electrons.
The oxidation state of an element shows the number of electrons of an atom of
the element that is involved in bond formation with the atom of another element.
An acid is any substance that increases the hydronium (or oxonium) ion (H3O+)
concentration in solution.
There are Arrhenius, Bronsted-Lowry and Lewis acids depending on definition.
Acids may be divided into strong acids and weak acids.
Acids have a number of characteristics. They can also be classified as organic and inorganic acids.
A base is a substance that increases the hydroxide ion (OH-) concentration in
water.
There are Arrhenius, Bronsted-Lowry and Lewis bases depending on definition.
Bases may be divided into strong bases, weak bases, super bases and solid
bases.
Bases have a number of characteristics. A soluble base is known as an alkali.
The basicity of an acid shows how many replaceable hydrogen ions there are in
one molecule of the acid.
The PH scale is a graduated measure of how acidic or alkaline a substance is.
Neutralization reactions are of the form;
Chemistry can be found in all things; including MMA fights.
The collision theory describes the underlying processes that occur in chemical reactions.
Factors that affect the rate of chemical reactions are Reactant concentration, state of reactants/surface area, nature of reactants, temperature and catalyst
effects.
150
MNEMONICS
There is so much to remember here. Think up awesome, easy to remember mnemonics
as usual. Make it personal if you like. I’ll try to suggest some.
The types of chemical reactions:
N
S
P
C
D
R
R
T
C
D
C
N – Neutralization reaction
S- Solid-State synthesis
P- Precipitation reaction
C – Combustion reaction
D- Displacement reaction
R-Redox reaction
R-Reversible reaction
T-Thermal dissociation reaction
C-Catalytic reaction
D-Decomposition reaction
C-Combination reaction
What have you come up with? I love animals so naturally I thought of ‘No Sane People Can Drive Red Rabbits To Catch Dancing Cats’. Don’t think about it too much. I don’t even think sane people should catch random dancing cats let alone drive red rabbits.
Just enjoy how insane it is as that aids memory and then recall the mnemonic during exams or whenever you need it.
Factors that influence reaction rates:
N
S
C
T
C
N – Nature of reactants
S- State of reactants/surface area
C- Concentration of reactants
T – Temperature
C- Catalyst effect
I thought of ‘No Students Care To Combust’; As it should be. The last thing we want is having combusting students and that’s why you have such strict supervision in the laboratory because as you may have learnt by now, with chemistry almost anything is possible. Don’t forget to make this personal if you must so you can remember better.
151
REVISION QUESTIONS
1. Define chemical kinetics.
2. List and explain eleven types of chemical reactions.
3. Explain the collision theory
4. What are the factors that affect the rates of chemical reactions?
5. A substance X reacts to form 25g of oxygen gas at a pressure of 40 atm at
standard temperature.
a) Calculate the volume of the O2 produced.
b) What is the molarity of O2?
6. Determine the oxidation states of the following;
a) S in H2SO4
b) P in NaH2PO4
c) Na in Na2O2
d) Fe in FeCl2(oxidation state for Cl in the compound is -1).
e) Sb in Sb4O6
152