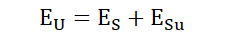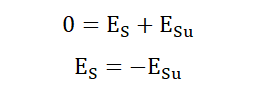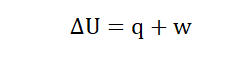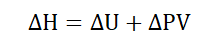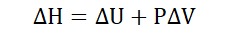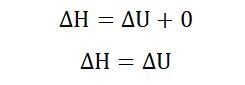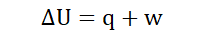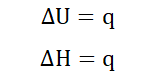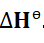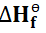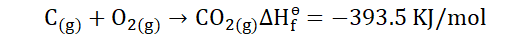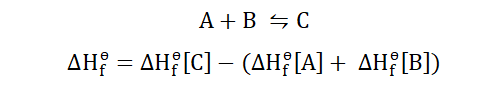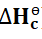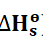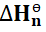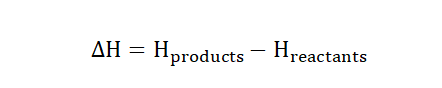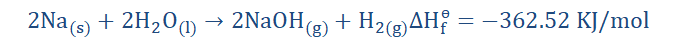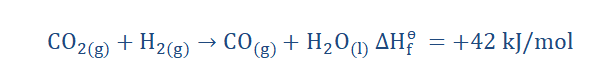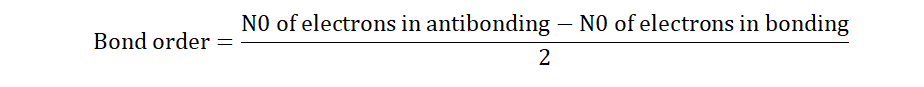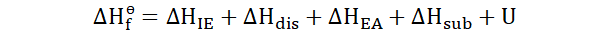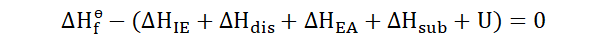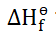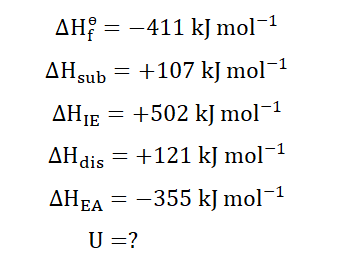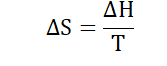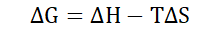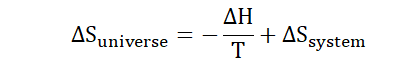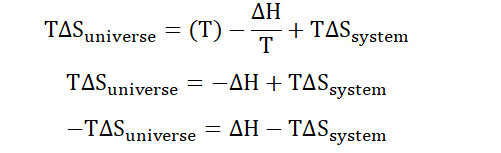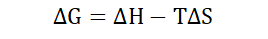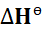CHAPTER 6: MORE ON REACTIONS
Energy Changes
Energy is known simply as the capacity to do work. This means that whatever contains energy can expend it in some way to achieve an aim. In chemical reactions there is usually a difference in energy between reactants and products. When we talk about energy in chemistry we usually try to understand the energy interactions between
reactants and products. All matter contains energy. When you eat food you obtain
energy from the food and become able to carry out your activities. I can only type this long because I ate a hearty dinner. You can only sit so long for classes because you consume egusi soup, yams, pasta, rice or whatever it is that you eat that is of course edible. Your generators work because they use up the energy stored in the form of 153

petrol. Cars also run because they have fuel- which is energy-stored in them. Energy is all around us and as we have always done with things that are all around us we put on our deerstalker hats and become academic Sherlocks to deduce their underlying
chemistry.
Chemical thermodynamics
Chemical thermodynamics is a field of chemistry that deals with the study of how energy changes impact chemical reactions. It can also be defined as the study of the
interactions between heat and work in a chemical system. There are three
fundamental laws of chemical thermodynamics that guide these energy interactions in chemistry.
The first law of thermodynamics
This is also known as the law of conservation of energy and states that energy can only change from one form to another but can never be created or destroyed. This law therefore implies that the total energy in the universe is constant. A good way to visualize this law is to picture a relay race. Imagine that the different runners on the field are the different energy forms and that the baton is the energy. In a standard relay race as one runner reaches the next he or she passes on the baton. Only a single baton is required in the duration of time for the race and no new batons are added or removed until the race is concluded. The law of conservation of energy is somewhat similar.
Energy changes form just as in the case of the runners but it’s the same amount of energy as in the baton in that regulated and closed environment; that environment can be taken to be the track.
154
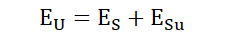
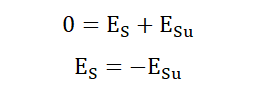
Figure 6-1 We can compare the first law of thermodynamics to running a relay race.
The law can be represented quantitatively in the following way;
EU is the energy of the universe, ES is the energy of a specific system and ESu
is the energy outside that system; all the other energy in the universe. So we
see that the total energy in the universe can be taken to be the energy of
whatever system is under consideration in addition to the energy surrounding
that system, outside it.
We said that the energy in the universe is constant. This implies;
This last equation shows that whatever we do to the energy system affects
the energy of its surroundings. If we subtract from the energy of a system we
add to the total energy in the surroundings and vice versa. It is an important
concept and a succinct representation of the first law of thermodynamics.
But what kind of energy are we talking about? There are many forms of
energy in the universe, but when we boil them all down we realize that there
are only three main types of energy. We know these types of energy as
kinetic, potential and internal energy.
Kinetic energy is defined as energy due to motion. In order to make a
football move on the field, footballers kick the ball. They apply a force to the
ball and so are said to do work on it. This work is then gained in the form of energy by the ball. The faster the ball moves, the greater the energy. There
would be more kinetic energy gained by a football kicked by Ronaldo than
one kicked by me on account of the fact that Ronaldo would as a result of
practice place sufficient force on the ball, hence more work would be done on
it and consequently more kinetic energy obtained.
Potential energy on the other hand is defined as the energy due to the
position of a body relative to others. It can also be defined as energy at rest.
Relative to a person on the ground, a person standing on the balcony of a tall
building has greater potential energy. This is because the person above has a
greater capacity to do work on the occasion that he falls; but we hope he
won’t.
155
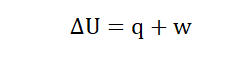
Atoms and molecules possess potential and kinetic energies. We have
thoroughly explored how molecules possess and utilize kinetic energy. Now
however our main concern is the third type of energy as it concerns chemical
reactions the most.
Internal energy (U)
This energy type concerns the positions of elementary entities as well as their
interactions. These interactions take the form of translations, vibrations and
rotations. Translational motion of micro particles involve a change in the
object’s position; as in moving from one point in space to another. Vibrations
are more repetitive motions of particles and rotations are akin to circular
movement around a point. These motions in addition to potential energies
make up the internal energy of a micro particle system.
Unlike measuring the kinetic and potential energies of macro bodies, directly
measuring energy at an atomic level falls on the scale of difficult to
impossible. On account of this, chemists measure estimates of this energy by
measuring internal energy changes that occur in the system instead. The
change in internal energy can then be obtained by applying the formula;
Where ΔU = Change in internal energy
q = Heat transferred to or from the system
w= Work done to or by the system
In the event that heat is lost from a system into the surroundings, q is
accompanied by a ‘-‘ sign to show this. When a system gains heat from the
surroundings it is denoted +q.
In the same way if work is done by the system on the surroundings, w
becomes –w. In the reverse of the situation; when the surroundings do work
on a system it becomes +w.
The unit of energy is the joule.
156
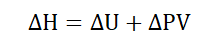
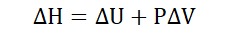
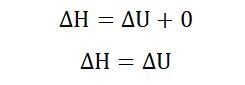
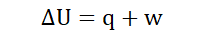
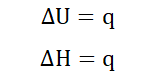

Enthalpy
For a system existing at some given pressure and volume, the enthalpy of
that system is defined as the sum of its internal energy and the product of its
pressure and volume. Enthalpy therefore comprises the entire heat content of
a system. It shows how much heat is either absorbed by a system or released
into its surroundings. It is usually shown as a change in enthalpy to account
for changes in energy between one state and another, such as between
reactants and products.
Where ΔH = Change in enthalpy
ΔU = Change in internal energy
ΔPV = Change in the product of pressure and volume
In systems of constant pressure, the formula changes to give;
Since the pressure is constant;
Recall that;
With constant temperature and pressure no work is done to or by the system
and so w become 0 hence;
At constant temperature and pressure, the enthalpy of a system is therefore
equal to the heat absorbed by or given off by the system. This shows that at
constant pressure in the event a system gives of heat to the surroundings and
hence has a negative value, the enthalpy of that system would also be
negative. When the system absorbs heat and has a positive value as a result,
the enthalpy assumes a positive value as well. For a chemical reaction the
enthalpy content can be obtained by subtracting the enthalpy of the reactants
from the enthalpy of the products.
157
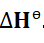
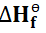
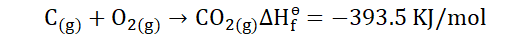
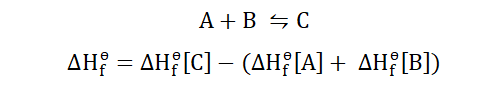
Standard Enthalpy
In order to allow for more accurate measurements, scientists set up a
standard that is used in energy calculations and experiments regarding
chemical reactions.
“The standard enthalpy of a reaction is defined
as the enthalpy change that occurs when
molar amounts of substances undergo
chemical reactions under standard conditions.”
It is denoted by
.
The standard conditions mentioned here mean that the temperature is at 298
k (25◦C), the pressure is 100 kPa (another unit for pressure) and the
substances involved have concentrations of 1 mol dm-3. Standard enthalpy
changes vary depending on the type of reaction considered. They are
standard enthalpies of formation, combustion, solution and neutralization.
Standard enthalpy change of formation(
)
The standard enthalpy of formation of a substance is defined as the enthalpy
change that occurs when a mole of the compound is formed from its elements
under standard conditions.
To understand this, let’s consider the reaction below.
This shows that heat is lost in the formation of the product and that the
standard heat of formation of CO2 is -393.5 KJ/mol. In order to know how to
solve for the heat of formation we would need to know the standard
enthalpies for the product and reactants. For;
158
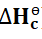
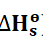
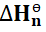
In order to easily arrive at the standard enthalpies we would have to apply a
special formula which we would discuss shortly.
Standard enthalpy change of combustion(
)
It is no surprise that combustion concerns the release of relatively large
amounts of energy in the form of heat.
The standard enthalpy change of combustion is therefore defined as the
enthalpy change that occurs when one mole of a substance burns completely
in an oxidant (usually oxygen) under standard conditions.
Just as we saw in the standard heat of formation, the standard heat of
combustion is dependent on the same standard conditions of temperature,
pressure and molarity.
Standard enthalpy change of solution(
)
Lattice energy is known as the energy required by the molecules of solvents to break up bonds of ionic solutes so as to allow dissolution. Hydration energy is the energy then required to hydrate the split up ions. These two process
allow for the release or absorbtion of heat by the solution.
The standard enthalpy change of solution is the enthalpy change that occurs
when one mole of a substance dissolves completely in a solvent to form a
solution under standard conditions.
Standard enthalpy change of neutralization(
)
Heat changes in neutralization reactions usually involve the release of energy
as acids and bases combine to form salt and water.
The standard enthalpy of neutralization of a reaction is defined as the
enthalpy change that occurs when when one mole of H+ from an acid react
with one mole of OH- from an alkali to form a mole of H2Ounder standard
conditions.
159
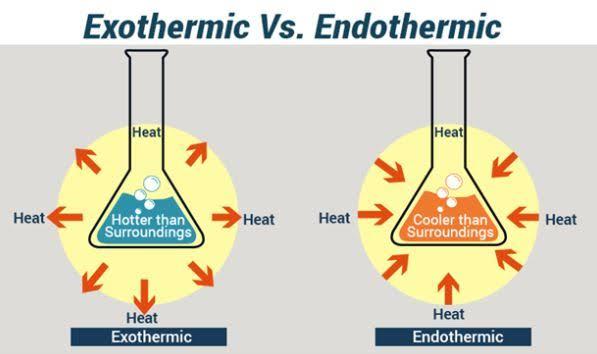
Exothermic and endothermic reactions
Chemical reactions involve the evolution or absorption of heat. In many cases
both heat removal and gain could occur for a single reaction. However there
is always a net process that favors one of the processes more. When a
reaction involves the release of heat energy to the surroundings it is termed
an exothermic reaction. When it largely involves absorbing heat from the
surroundings it is an endothermic reaction. The reaction vessel of an
exothermic reaction is hotter than its immediate surroundings, while the
reaction vessel of an endothermic reaction is colder than its immediate
surroundings.
“An exothermic reaction is one that involves
the release of heat from a reaction system into
the surroundings.”
“An endothermic reaction or process involves
the gaining of heat from the surroundings to a
reaction system.”
160
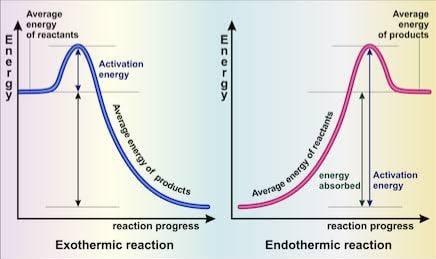
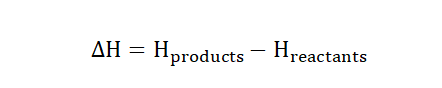
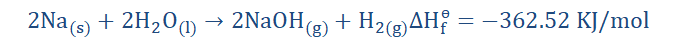
Figure 6-2 Energy level diagrams of exothermic and endothermic reactions.
We know from the collision theory that effective collisions between reactant
particles give rise to products. We also know that the particles must overcome
an energy barrier and possess the activation energy needed to form products.
In an energy level diagram, this activation energy is shown to be the
difference between the energy of the reactants and the energy of the
products. For an exothermic reaction the energy of the reactants exceeds the
energy of the products. The reaction therefore demands less energy input but
releases excess energy in the form of heat into the surroundings. It also
explains why ΔH is negative when applied to the equation;
For an endothermic reaction, the energy of the products surpasses that of the
reactants. More energy is required from the surroundings to exceed the
energy barrier and arrive at the activation energy so that products can be
formed. This energy is usually in the form of heat. ΔH is positive in
endothermic reactions for the same reason it is negative in exothermic
reactions and so we can tell whether a reaction is exothermic or endothermic
by noting what sign accompanies the ΔHɵ. For instance;
This reaction is exothermic as we can see the negative sign of ∆Hɵf showing
that heat has been evolved. A large amount too! This makes sense when we
consider the vigorous nature of the reaction between sodium and water.
161
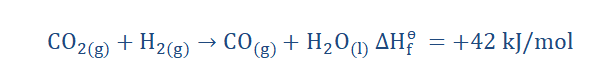
In the equation above we note a positive ∆Hɵf and so we can tell that the
reaction is endothermic.
Reactions and Bond energies
We’ve been talking about the structures atoms form for quite some time.
When atoms of elements come together they form such wonderful
compounds but how do they hold together? Imagine a bowl of popcorn. You
are watching your favorite episode of BBC Sherlock or Africa Magic’s The
Johnsons when your imaginative mind wonders what honey and popcorn
would taste like. You decide to do just that but after a while you find out that
the individual popcorn pieces stick together. The honey acts as some kind of
‘glue’ holding the popcorn together. It would never have been possible for the
popcorn to stick together without this makeshift ‘glue’.
We can compare that to bonds and bond energy. Bonds must exist between
atoms to hold them together. These bonds require energy to keep them so as
well as energy to break them apart. The energy required by compounds to
hold atoms of their elements together or break them apart is termed bond
energy.
“Bond energy is defined as the energy
required for the breaking or forming of atomic
bonds.”
When the bond existing between atoms is strong, greater energy would be
required to break them apart. They are therefore said to have high bond
energies. On the other hand, when the bond existing between them is weak,
less energy would be needed to split them and so they possess low bond
energy. A H-H (hydrogen to hydrogen) bond for instance has an average
bond energy of 432 kJ mol-1 as opposed to an I-Br (Iodine to bromine) bond
of only 175 kJ mol-1. Also, a C≡N (carbon to nitrogen triple bond) has average
162
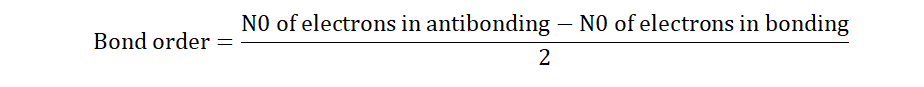
bond energy of 891 kJ mol-1 while a C=N (carbon to nitrogen double bond)
has bond energy of 615 kJ mol-1.
Two other concepts accompany bond energy and these are bond order and
bond length.
Bond order shows the number of pairs of electrons that engage in bond
formation between two atoms. For C-C for instance, we know that two pairs of
electrons-one donated by each atom-is involved in bond formation between
them (remember that a ‘dash’ is equivalent to an electron pair). The bond
order is therefore 1. In a doubly bonded carbon to carbon pair of atoms (C=C)
we see that we now have four pairs of electrons; hence two bonds. The bond
order here becomes 2. What would the bond order be for a triple bond? How
about no bonds at all? I’ll wait.
The general formula for finding bond order is;
The antibonding electrons consist of those electrons that are not involved in bond formation, while the bonding electrons are those that are.
Bond length exists in inverse proportion to bond order. This means that
when bond order is increased, bond length falls and vice versa. When the
number of bonding electrons between atoms is increased, there is a greater
pull or force of attraction between them and consequently the length of the
bond between them shortens or reduces. It is measured in picometers. The
greater the number of picometers between atoms, the lower the bond order.
Single bonds therefore have greater bond lengths than triple bonds and triple
bonds have greater bond order than single bonds.
Hess law of heat summation and the Born-Haber cycle
Remember when I promised to show you a relatively easy way to calculate
enthalpy without having to memorize the enthalpies of all substances? Well
this is what I was referring to. We would be applying the Born-Haber cycle
and Hess law of heat summation to achieve this. The Born-Haber cycle was
put forward by two scientists; Max Born and Fritz Haber. Another scientist
named Kasimir Fajans had independently formulated it and so I find the
163
concept to be more accurately identified as the Born-Haber-Fajans cycle.
That’s really long so maybe we’ll stick with Born-Haber.
Hess law explains that the total enthalpy change of a reaction is actually the sum of the enthalpy changes of individual processes involved in the
reaction. The Born-Haber cycle is an aspect of Hess law that specifically concerns ionic substances. The cycle attempts to explain why ionic solids are
so stable and why these solids despite similar structures differ greatly from
each other in terms of physical characteristics. It has been shown that
enthalpy is not enough to account for these. In order to do so the concept
oflattice energywas introduced. We had spoken of lattice energy earlier
when we defined the standard enthalpy change of formation. It turns out that
lattice energy is the reason for the stability of ionic solids as well as explains why their physical characteristics such as solubility differ so much.
Lattice energy can be defined in two ways. It can be defined as the energy
required by an ionic substance to split it apart into simpler gaseous
constituents. Since it requires the addition of energy here this process is endothermic. It is also defined as the loss of energy usually in the form of
heat when an ionic substance is formed by simpler gaseous constituents. As you can probably tell this process is exothermic.
In order to truly understand the Born-Haber cycle we have to examine five
enthalpies that accompany most chemical reactions. These are very, very
briefly explored below. They would be explained more thoroughly later.
Ionization energy: This is the amount of energy needed to remove an
electron from a neutral atom. It is denoted by ΔHIE.
Dissociation energy: This is the energy needed to break a substance apart
into its constituents. It is denoted by ΔHdis.
Electron affinity: This is the amount of energy needed to remove an electron from a neutral atom. It is denoted by ΔHEA.
Sublimation energy: This is the amount of energy needed by a substance to
change its state from solid to gaseous without passing the liquid state. It is
denoted by ΔHsub.
Internal energy: Do we need to explain this one? Yes? Alright. This is the energy possessed by a body due to the heat associated with it and the work
164
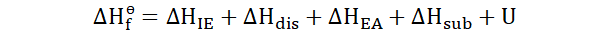
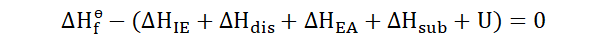
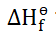
done on it or by it. It is denoted by U. When ionic solids are concerned the
internal energy becomes equal to the latent energy.
In order to analyze the Born-Haber cycle of a reaction we add up these
energies and find out the total enthalpy change. As an example, if we need to
find out the total enthalpy change for the formation of a substance the
equation for the Born-Haber cycle would be taken as;
This is also:
Let’s try to apply the cycle to an actual problem so we don’t seem like we’re
speaking some cryptic language. Let’s apply this to the formation of table salt;
NaCl.
The formation of NaCl is divided into a number of stages. The enthalpies of
these stages can be measured but as we stated earlier the lattice energy
cannot. The
for Nacl for instance can be experimentally derived and is
gotten as -411 kJ mol-1.
The first stage of the formation of NaCl is sublimation, where the solid Na
sublimes into atomic gaseous, simpler form. The process here is
endothermic since heat is required to achieve this.
When this is concluded, each neutral atom then releases the single valence
electron on its valence shell. In doing so, they release energy which we know
as ionization energy.
Dissociation then occurs as molecular chlorine (Cl2) splits into single atoms.
The process is endothermic as energy is absorbed to achieve this.
Remember the electrons given away by the individual Na atoms? The Cl
atoms accept the electrons. Energy in the same magnitude as the electron
affinity is evolved by this exothermic process.
Finally, the two gasses composed of individual ions combine to form their
solid offspring; the NaCl molecules. Energy is being released which we know
as lattice energy.
Did you count the number of processes involved here? Who would have
thought that the single reaction had so many levels to it?
165
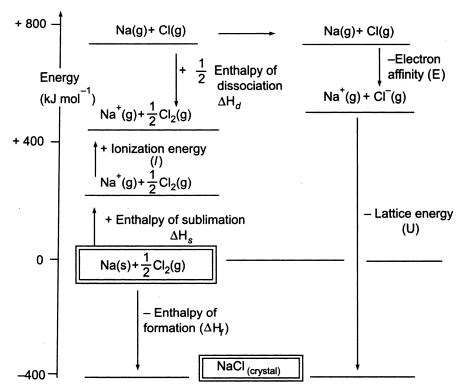
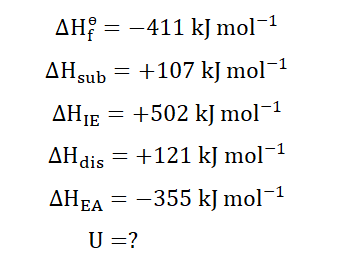

Figure 6-3 Born-Haber cycle for the formation of NaCl.
The above is the Born-Haber cycle for the process we have explained above.
Now we would apply the associated formula to find the lattice energy for the
reaction.
Experimentally the following enthalpies for the formation of NaCl have been
derived;
Inputting these into the equation;
166

And now we see that the lattice energy of the formation of NaCl is -786 kJ mol-1. Isn’t it just wonderful how we can determine this without leaving our desks? You can thank Germain Hess, Max Born, Fritz Haber and Kasimir Fajans for that.
Entropy (ΔS)
Figure 6-4
I was never a destructive kid but this was certainly one of my more interesting
topics. I just felt that I didn’t have to think too hard to get it. It just made sense.
Order might be harder to spot but disorder? Well that’s everywhere and in
more ways than one too. The idea of entropy, put quite simply concerns how
disordered a system is.
“Entropy is a thermodynamic property that
describes the degree of disorder of a system.”
Entropy simply tells you how spread out elementary particles are; how
inconsistent their arrangement is meaning the extent to which they deviate
from consistency. It is denoted by S. When considering initial and final states
we use ΔS instead so as to show that the entropy of the system has changed.
From this definition, can you tell what has the highest entropy between a
solid, liquid and gas? I’ll wait.
If you truly thought about that you’d realize that substances in the gaseous
state tend to have greater ‘randomness’ or ‘disorder’ than substances that are
liquids or solids. It is therefore no surprise when we learn that entropy is
dependent on temperature; meaning that the greater the temperature of a
167
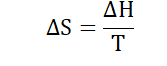
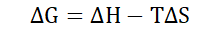
system the greater the disorder and vice versa. At a constant temperature
entropy is related to enthalpy in the following way:
Gibbs free energy (ΔG)
Josiah Willard Gibbs in 1873 published papers that showed that the direction
of a chemical reaction could be predicted. By utilizing his new found study
scientists could tell if a reaction was spontaneous or non-spontaneous.
Spontaneous reactions do not require added energy to proceed while non-
spontaneous reactions needed external energy pumped into them in order for
them to take place. Gibbs’ equation combined enthalpy and entropy into a
single equation and he identified this direction of a reaction or its capacity to do work as available energy. But today we know it as free energy.
“The free energy of a chemical reaction is
defined as a measure of its available energy to
do work.”
Free energy is quantitatively equivalent to the subtraction between the
enthalpy of a system and the product of its entropy and temperature. It is
usually presented as a change in free energy (ΔG) under standard conditions
(at constant temperature and pressure).
168
The second law of thermodynamics
Figure 6-5 Time moves in one direction
Have you ever wanted to travel in time? Maybe back to when you were little
or perhaps to right some wrongs or steal Einstein’s work and come back to
the present so you can claim it instead! Maybe you should not attempt the last
one but for the first two I imagine you may not mind that if it was a pleasant
time for you. In that case allow me to tell you that you just can and it’s fairly easy; so easy that I can say it in just six words- break the second law of
thermodynamics.
“The second law of thermodynamics states
that for an isolated system, the entropy is
always increasing and so never decreases.”
What does time have to do with entropy? I’ll show you. In nature there is
always an increase in disorder. This fact can only be negated if systems are
in a state of continuous reversibility; meaning that every occurrence would be
reversed. This is however not how the universe works. Most natural
occurrences are irreversible. This is because systems in the universe are
attempting to achieve thermodynamic stability. The second law is the reason
why each time you clean up your room it gets messy again. No matter how
neat you are and what systems you put in place, it does. I may not have been
the tidiest child but I was sensibly tidy and yet once upon a time I was
convinced there was a monster under my bed who took some sick pleasure in
messing up my room. Another instance can be found in ice blocks. Why do
they always melt when left on their own but never freeze up even more? Why
does heat flow from a hotter body to a colder one and not the other way
169


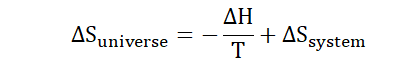
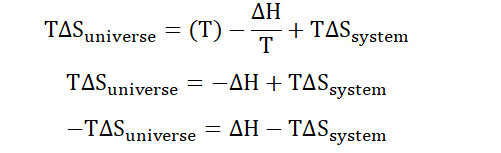
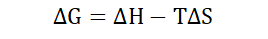
around? The second law tells us that disorder is always increasing. It is a one
way street and there are no U-turns. As the universe is a sort of isolated
system, time is no exception to this rule. It is subject to ever increasing
entropy and so proceeds in only one way. The next time you watch the movie
‘Back to the Future’ for the hundredth time and wonder why we can’t travel
back in time, blame the second law of thermodynamics. It can be expressed
as;
Now we know what this law is, how does it apply to chemical reactions?
The second law can be restated as;
“For an isolated spontaneous system, the
entropy is always increasing.”
We had mentioned spontaneity before now when we spoke of free energy.
The second law can be described in terms of free energy, entropy and
enthalpy as we would soon see.
Substitute into the equation;
Multiply both sides of the equation by T;
-TΔS is the free energy ΔG that we discussed earlier. We can therefore see how the second thermodynamic law relates to the free energy of a system.
170
The third law of thermodynamics
The third law is concerned with absolute zero. You may recall that we have mentioned absolute zero a few times; for instance when we spoke of the Bose-Einstein condensate. It is the lowest possible temperature that can be reached by a body. Below this temperature motion ceases. This law asserts that as
temperature approaches the absolute zero which is equivalent to 0 K, entropy
tends to a constant value hence motion ceases since entropy becomes 0. In
some academic circles it is known as Nernst’s law since it was stated by the chemist Walther Nernst between the years of 1906 to 1912.
The laws of thermodynamics appear to me to be a perfect story of
thermodynamics. We begin with a fundamental universal principle that the
energy of the universe is constant and cannot be altered since it is to the best of our knowledge a closed system. We then proceed to learning that this same
universe will always have a continuous increase in disorder and finally we learn
that in approaching absolute zero, the entropy would become constant and
motion will cease. We see the beginning, growth and death of nature in just a few pages.
The last paragraph was just to prove that chemists can be poetic too. It’s that
time again for some well deserved rest. When you return, it’ll be the usual.
171
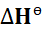
SUMMARY
Energy is the capacity to do work.
There are three types of energy: potential, kinetic and internal energy.
Potential energy is energy due to position, kinetic energy is energy due to motion and internal energy is energy due to the positions and interactions of elementary entities.
Chemical thermodynamics is a field of chemistry that deals with the study of how energy changes impact chemical reactions. There are three laws of chemical
thermodynamics.
The first law of chemical thermodynamics states that energy can neither be
created nor destroyed.
The enthalpy of that system is defined as the sum of its internal energy and the product of its pressure and volume.
The standard enthalpy of a reaction is defined as the enthalpy change that
occurs when molar amounts of substances undergo chemical reactions under
standard conditions. It is denoted by
.
There are standard enthalpies of formation, solution, neutralization and
combustion.
An exothermic reaction is one that involves the release of heat from a reaction system into the surroundings while an endothermic reaction or process involves
the gaining of heat from the surroundings to a reaction system.”
Bond energy is defined as the energy required for the breaking or forming of
atomic bonds.
Bond order shows the number of pairs of electrons that engage in bond formation between two atoms.
Bond length exists in inverse proportion to bond order. This means that when
bond order is increased, bond length falls and vice versa.
Hess law explains that the total enthalpy change of a reaction is actually the sum of the enthalpy changes of individual processes involved in the reaction.
The Born-Haber cycle is an aspect of Hess law that specifically concerns ionic substances.
Lattice energy can be defined as the energy required by an ionic substance to
split it apart into simpler gaseous constituents. It can also be defined as the loss 172
of energy usually in the form of heat when an ionic substance is formed by simpler gaseous constituents.
Ionization energy is the amount of energy needed to remove an electron from a
neutral atom. It is denoted by ΔHIE.
Dissociation energy is the energy needed to break a substance apart into its
constituents. It is denoted by ΔHdis.
Electron affinity is the amount of energy needed to remove an electron from a
neutral atom. It is denoted by ΔHEA.
Sublimation energy is the amount of energy needed by a substance to change its state from solid to gaseous without passing the liquid state. It is denoted by
ΔHsub.
Entropy is a thermodynamic property that describes the degree of disorder of a system.
The free energy of a chemical reaction is defined as a measure of its available energy to do work.
The second law of thermodynamics states that for an isolated system, the
entropy is always increasing and so never decreases.
The third law of thermodynamics states that as temperature approaches the
absolute zero which is equivalent to 0 K, entropy tends to a constant value hence motion ceases since entropy becomes 0.
173
MNEMONICS
Did you find anything that made you feel the need for a mnemonic today?
The standard enthalpies:
S
C
N
F
S– Solution; as in “Standard enthalpy of solution. ”
C- Combustion; as in “Standard enthalpy of combustion. ”
N- Neutralization; as in “Standard enthalpy of neutralization. ”
F – Formation; as in “Standard enthalpy of formation. ”
‘Sandwiches Can Now Fly.’ No comments.
174
REVISION QUESTIONS
1. What is Chemical Thermodynamics?
2. List and state the three fundamental laws of chemical thermodynamics.
3. What is energy?
4. List and define the three types of energy.
5. Define the following:
a) Enthalpy
b) Standard enthalpy
c) Standard enthalpy of formation
d) Standard enthalpy of neutralization
e) Standard enthalpy of solution
f) Standard enthalpy of combustion
g) Bond energy
h) Bond order
i) Lattice energy
j) Bond length
k) Ionization energy
l) Dissociation energy
m) Sublimation energy
n) Electron affinity
o) Entropy
p) Free energy
6. Differentiate between exothermic and endothermic reactions.
7. State Hess’ law.
8. Explain with the aid of a diagram the Born-Haber cycle.
9. Why is it nearly impossible to move backwards in time?
175