6. STRATEGY
Strategy Analytics
Agents: system analysts, business analysts, scientist, engineers, technology management consultants;
Strategic moves : Focus on emerging healthcare, life science, biotechnology, pharmaceutical technologies.
 Call deep analytics ‘7-S’ model; explore how to ensure a perfect fit among 7- S elements – scope, system, structure, security, strategy, staff-resources, skill- style-support;
Call deep analytics ‘7-S’ model; explore how to ensure a perfect fit among 7- S elements – scope, system, structure, security, strategy, staff-resources, skill- style-support;
 Define a set of security goals and emerging technologies accordingly.
Define a set of security goals and emerging technologies accordingly.
 Do SWOT analysis: strength, weakness, opportunities and threats of existing technologies as compared to emerging technologies;
Do SWOT analysis: strength, weakness, opportunities and threats of existing technologies as compared to emerging technologies;
-
- Fair and rational business model innovation
- Who are the consumers?
- What should be the offering of products and services?
- What do the consumers value?
- How to deliver values to the consumers at rational cost?
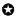 Do technology life-cycle analysis on ‘S’ curve : presently at emergence phase of ‘S’ curve.
Do technology life-cycle analysis on ‘S’ curve : presently at emergence phase of ‘S’ curve.
 Explore technology innovation-adoption-diffusion strategy.
Explore technology innovation-adoption-diffusion strategy.
-
- Cancer prevention strategies
 Proactive approach
Proactive approach
 Reactive approach
Reactive approach
 Deep learning
Deep learning
 Intelligent reasoning
Intelligent reasoning
-
-
- Case based reasoning
- Perception common sense reasoning
- Regenerative medicine
- Precision medicine
- Cancer genomics
 Biomedical technology
Biomedical technology
 Explore innovation model and knowledge management system for creation, storage, sharing and application of knowledge.
Explore innovation model and knowledge management system for creation, storage, sharing and application of knowledge.
 Adopt ‘4E’ approach: envision, explore, exercise and extend.
Adopt ‘4E’ approach: envision, explore, exercise and extend.
Dr. Muller and Dr. Nil Harvey are analyzing the strategy of innovation, adoption and diffusion of emerging healthcare technologies; it can be analyzed from different dimensions such as proactive self-healing approach, reactive approach, deep learning algorithm, intelligent reasoning and biomedical instrumentation. Intelligent reasoning should be explored in terms of case based reasoning, perception and common sense, logical and analytical reasoning and rational payment function. It is essential to adopt a set of reactive strategies such as alternative, integrated, regenerative and precision medicines to fight against cancer. It is rational to evaluate strength, weakness, opportunities and threats for various strategic options. The evolution and diffusion of cancer prevention technology depends on R&D policy, organization learning, knowledge management strategy and technology life-cycle analysis. An intelligent R&D policy should be explored through shared vision, goal and strategic alliance, collaborative and collective intelligence. There are various strategies of learning such as learning-by-doing and learning-before-doing. Learning-by-doing is effective in cancer care through deep learning on big data; it is also essential to attain deep practical and theoretical knowledge on cancer therapy through experimental medicines. In fact, it is clear from the aforesaid case analysis that different types of cancer demand different types of prediction and prevention technologies, best practices and therapies.
Technology trajectory is the path that the cancer prevention technology takes through its life-cycle from the perspectives of rate of performance improvement, rate of diffusion and rate of adoption in cancer care. It is really complex to analyze the impact of various factors on the trajectory of cancer prevention technology today. From the view of life-cycle, the technology of cancer prevention is at the growth phase of S-curve. The technology has evolved from the birth phase and going though growth phase. Initially, it may be difficult and costly to improve the performance of the new cancer prevention technology. The performance is expected to improve with better understanding of the fundamental principles and mechanisms of human biological system. Initially, the technology may be costly for the adopters due to various uncertainties and risks. Gradually, it is expected to be adopted by large segments of the market due to reduced cost and risks. The evolution of the technology is passing through a phase of turbulence and uncertainty; various entities are exploring different competing options of cancer care and a dominant therapy is expected to emerge through a consensus and convergence of the best practices. The dominant therapy must consider an optimal set of technological advancements which meet the demand of the cancer patients, cancer care experts, supply chain and design chain in the best possible way.
Let us consider the strategy of surgical operation for cancer care. It is essential to operate hard and soft tissues with proper care, precision, consistency, speed and control. The surgical operation is expected to be minimally invasive, less painful and faster healing. An innovative technological solution and surgical method for cancer care is expected to have higher success rate; lower recurrence rate, more precision, accuracy and effectiveness, less treatment time, faster recovery and healing, unmatched cutting, speed and control with high consistency and reliability, greater precision and control, efficiency and safety, less post operative problems due to minimally invasive procedure preventing damage to nearby tissues and less bruising, numbness and post operative pain, minimal invasion and no scars, simple OPD procedure, better patient experience and thus high credibility and high patient satisfaction. Laser technology is an emerging solution for the aforesaid desired surgical operation in cancer care.
Laser (light amplification by stimulated emission of radiation) is an effective solution as compared to conventional methods. Laser has a specific wavelength; it is focused in a narrow beam and creates a very high intensity light which is used for cutting through tissue in surgical operation. Laser therapy is used to destroy tumors or precancerous growths in skin, cervical, penile, vaginal, vulvar and lung cancer. It may be also used for cancer related to brain, prostate, piles, fissures, fistula and dental problems. Laser therapy can be used in standalone mode or in combination with chemotherapy or radiation therapy. It is used to relieve certain symptoms of cancer such as to remove a tumor blocking trachea or esophagus or colon polyps or stomach. It can also be used to seal nerve endings and to reduce pain after surgical operation and seal lymph vessels to reduce swelling and limit the spread of tumor cells. Laser therapy is given through a flexible endoscope fitted with optical fiber. It is inserted through mouth, nose, anus or vagina. Laser is then precisely focused to remove a tumor.
There are various types of laser therapies such as Laser-induced Interstitial Thermotherapy (LITT) and Photodynamic Therapy (PDT). Generally, CO2, argon and neodymium: yttrium-aluminum-garnet (Nd:YAG) lasers are used for cancer care. Laser therapy provides several benefits such as minimally invasive procedure, faster recovery, minimal recurrence, blood loss, pain and post operative discomfort and high success rate. Laser therapy demands specialized training of the surgeons and strict safety precautions. It is expensive; the effects may not last long and may be repeated for recovery.
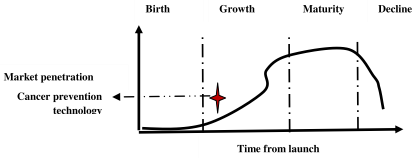
Figure 5.3 : Technology life–cycle analysis
Precision Medicine for Cancer Care : Let us exercise SWOT analysis on precision medicine; it is a a medical model having customization of healthcare practice in terms of medical decisions, treatments, practices, products and services being tailored to individual patient. Diagnostic testing is done for genetic, molecular and cellular analysis to select correct and optimal method of the treatment of a patient using a set of intelligent tools such as molecular diagnostics, imaging, and analytics. Precision medicine is basically tailoring of medical treatment to individual characteristics of each patient; not creation of drugs or medical devices unique to a patient, but rather the ability to classify individuals into subpopulations that differ in their susceptibility to a particular disease or in their response to a specific treatment. Preventive or therapeutic interventions can then be focused on those patients who will benefit considering a set of critical issues such as patient care cost and side effects of medicine. Genomic medicine is an emerging medical discipline that involves using genomic information about an individual as part of their clinical care (e.g. for diagnostic or therapeutic decision making) and the health outcomes and policy implications of that clinical use. P4 is an approach to make medicine more predictive, preventive, personalised and participatory. The basic objectives are to quantify wellness and predict and prevent disease; understand human health and moderate the course of chronic diseases, correct disabling physical conditions and cure molecular deficiencies; it demands the convergence of system medicine, digital technologies and consumer driven healthcare. Personalized medicine is a form of medicine that uses information on genomics i.e. person's genes, proteins and environment to prevent, diagnose and treat disease.
Precision oncology is the branch of precision medicine for cancer care based on molecular profiling tests and DNA sequencing. Artificial intelligence is providing a paradigm shift towards precision medicine to understand genotypes and phenotypes in existing diseases improve the quality of cost effective patient care and reduce mortality rates. Machine learning algorithms are used for genomic sequence analysis and drawing inferences using big data analytics. Health care service providers are expected to understand better the impact of environment, lifestyle and heredity on patient's health, disease or condition using the knowledge of precision medicine, They can predict correctly which treatments will be most effective, preventive and safe, There are other several benefits of precision medicine : shift from reaction to prevention, predict susceptibility to disease accurately, improve detection of disease, preempt progression of disease, customize prevention strategies of disease, prescribe more effective drugs, avoid prescribing drugs with predictable side effects, reduce the time, cost, and failure rate of pharmaceutical clinical trials, eliminate trial and error inefficiencies that may inflate health care costs and undermine patient care. One of the critical issues in cancer patient care is the side effects of chemotherapy. Precision medicine may be an interesting strategic move to cure cancer in future.
Cancer Genomics : Cancer is considered as a genomic disease since a normal cell becomes a cancer cell through successive genomic alterations. Molecularly Targeted Agents (MTAs) block a peculiar molecular genomic alteration in cell proliferation, angiogenesis, metastasis and invasion of tumor. Precision medicine analyzes information on genes, proteins, environment, location of tumor and histology of a patient to diagnose, treat and prevent cancer. Precision medicine is an emerging technology in oncology based on the concept of MTAs. It is important to analyze the effectiveness of precision medicine in terms of efficiency of bioinformatics algorithms, histology independent drug development, clinical trials to evaluate a treatment algorithm instead of drug efficacy, molecular genomic alterations across different types of tumor and molecular profiling of tumor of cancer patients.
RNAs are polymeric molecules which carry genetic information and used in protein synthesis; only a minor fraction of human genomes encode for proteins and the remaining large fraction of the transcripts are noncoding RNAs (ncRNAs). The noncoding RNAs are potential biomarkers and therapeutic targets to facilitate precision medicine in cancer care. It is an interesting research agenda to explore diagnostic, prognostic, biomarkers and therapeutic strategies of ncRNAs. ncRNAs are broadly classified into distinct classes based on the lengths, unique biogenesis routes, three dimensional structure and modes of action such as ribosomal RNAs, transport RNAs, small nucleolar RNAs (snoRNAs), microRNAs (miRNAs), small interfering RNAs (siRNAs), PIWIinteracting RNAs (piRNAs), hairpin RNAs (hpRNAs) and long noncoding RNAs. ncRNAs regulate gene expression through diverse mechanisms such as mediating imprinting, alternative splicing and modification. Deep sequencing technologies generate high throughput transcriptomic and demand the support of efficient bioinformatics mechanisms for proper storage, intelligent analysis, visualization and interpretation of data, RNA identification, structure modeling, functional annotation and network inference. US government invested $215 million to launch Precision Medicine Initiative (PMI) in 2015. It is a customized healthcare model which considers individual variability and tailors medical treatment to individual patient. Precision medicine is dependent on molecular diagnostics to select correct therapies based on genetic data of a patient.
Genomic information is getting used for the diagnosis of lung, breast and pancreatic cancer. The treatment of lung cancer is effective based on diagnostic, prognostic and predictive findings, microRNA profiling and high throughput sequencing. Molecular alterations and routine genomic profiling is getting applied for the treatment of lung cancer. Evolving technologies (e.g. next generation sequencing, high density microarrays and high throughput expression profiling platforms) enable genomic profiling at a reasonable cost. Pancreatic Ductal Adenocarcinoma (PDAC) is a lethal disease with the worst prognosis among all solid tumors. Genomic landscape has revealed that massive scale sequencing provides unprecedented opportunity to dramatically improve diagnosis and treatment of pancreatic cancer. Breast cancer is the most frequent female cancer. Genomics is effectively used for the treatment of breast cancer through proper hormone therapy.
Regenerative Medicine for Cancer Care : The basic objective of regenerative medicine is to develop methods for regrowing, repairing or replacing damaged or diseased cells, organs or tissues (William Haseltine,1999). It includes generation and use of therapeutic stem cells therapy, tissue engineering, molecular biology, cell transplantation, production of artificial organs and biomechanics prosthetics. It deals with the process of replacing, engineering or regenerating human or animal cells, tissues or organs to restore or establish normal function. It tries to engineer damaged tissues and organs by stimulating the body's own repair mechanisms to functionally heal previously irreparable tissues or organs. It includes the injection of stem cells obtained through directed differentiation (cell therapies); the induction of regeneration by biologically active molecules administered alone or as a secretion by infused cells (immunomodulation therapy); and transplantation of in vitro grown organs and tissues (tissue engineering). It is relatively a costly treatment. There are various types of regenerative medicine such as Stem cell therapy (Stem cells injected into bone, cartilage or fat cells to stimulate healing in the body), platelet rich plasma (prp), lipogems and prolotherapy. Stem cell injections may last upto one year. Stem cells may be effective in cancer care to repair, restore, replace and regenerate cells at the target location,
Regenerative medicine covers a range of treatments intended to repair or replace damaged cells, tissues, or organs such as cell therapies, bioengineered tissue products, and gene therapies. Stem cell treatment involves infusion of healthy stem cells into the patient through a painless process of stem cells transplantation through intravenous (IV) infusion. For bone marrow or blood stem cell transplant, engraftment takes 2-3 weeks; for cord blood transplant, it takes 3=5 weeks. Stem cell transplant has some negative side effects such as low blood cell counts, Graft- versus-host disease (GVHD), Veno-occlusive disease (VOD), digestive system problems. skin and hair problems. pain, kidney and lungs problem. The success rate is 82.2%. The basic objective of regenerative medicine is to provide safe and reliable ways to repair, restore or replace damaged tissues or organs. Cord blood and tissues are the building blocks of regenerative medicine. Natural stem cells extracted from embryonic, hematopoietic, mesenchymal or adult tissues) or induced progenitor stem (iPS) cells can be modified by gene therapy for use in regenerative medicine. Stem cell therapy is useful for regrowth of cartilage, knee pain and growth of hair. There are side effects such as temporary swelling and pain. Regenerative medicine is an interesting strategic option of cancer care in future.
Integrated Medicine for Cancer Care: Integrative medicine may be any type of medical practice or product that is not standard care. For cancer, standard care may be surgery, chemotherapy, radiation and biological therapy. Integrative medicine is complementary care used alongwith standard care. For cancer, it combines the best of both types of care. It is a combination of medical treatments for cancer and complementary therapies to cope with the symptoms and side effects. Complementary Alternative Medicine (CAM) may include acupuncture, yoga and meditation for treatment of cancer and scientific evidence supports this approach to health and healing. Can cancer be cured naturally? There is no evidence of alternatives of cancer cure. Alternative complementary therapies can interfere with chemotherapy or radiation and make them less effective or may have other negative effects. Integrative medicine combines complementary treatments with conventional care. Conventional medicine relies on methods proved to be safe and effective with carefully designed trials and research. But, many complementary and alternative treatments lack solid research for sound decisions. What are the benefits of integrative medicine? It is a comprehensive approach to care i.e. treating the patient through a holistic approach; not just the condition or disease; setting the foundation for overall health, This method may reduce the cost of cancer care. But, it may have negative effects or even risks of death. What is the best treatment for cancer? In fact, cancer care may be done effectively through a combination of treatments such as surgery with chemotherapy and/or radiation therapy, immunotherapy, targeted therapy or hormone therapy, precision medicine, regenerative medicine and integrated medicine.
Dr. Muller is analyzing the strategy of innovation, adoption and diffusion of biomedical technologies. This element can be analyzed from different dimensions such as R&D policy, learning curve, SWOT analysis, technology life-cycle analysis and knowledge management strategy. An intelligent R&D policy should be defined in terms of shared vision and goal,. Biomedical innovations are closely associated with various strategies of organization learning and knowledge management. The aforesaid biomedical innovation is closely associated with R&D policy and organizational learning strategies in new product development. There are various strategies of learning such as learning by doing and learning before doing. Learning before doing is possible through laboratory experiments, prototype testing and simulation. Deep practical and theoretical knowledge can be achieved through laboratory experiments. Learning by doing is also important to understand the impact or side-effects of the implantation of biomedical devices and oral insulin.
Technology innovation on biomedical technology is associated with various strategic moves and functions such as scope analysis, requirements engineering, quality control, product design, concurrent engineering, talent management, team management and coordination, resources planning, defining products specification, concept development, concept evaluation, system architecture design, detailed design, production plan development, roll out, prototyping, testing, documentation, tools development and regulatory issues [ 37-38].
The design of a medical product is a complex task having uncertain information, high stakes and conflicts, varieties in size, scope, complexity, importance and cost and various constraints in terms of product quality, product cost, development cost, development time and development capability. It is essential to adopt concurrent engineering approach which considers all aspects of the problems faced and a clearly defined role. It is challenging to develop a product development team with specific skills and group dynamics. The next step in our process is to transform these needs into specifications that can be used to guide the design decisions. These product specifications become the targets for the product development. The concept development phase is the time to search out ideas that will meet the need. Next important steps are concept evaluation through SWOT analysis, design of system architecture, prototyping and product testing. Finally, it is essential to satisfy various issues of regulatory compliance such as approval of FDA. It is not a simple task to make rational decisions on strategic technologies, ICT, technology infrastructure development and renewal of existing technologies.
SWOT Analysis: It is an interesting research agenda to analyze strength, weakness, opportunities and threats of innovation on biomedical technology. Strength indicates positive aspects and benefits; weakness shows negative aspects and limitations of the same; opportunities explore the growth potential and threats assess the risks of the technology. Let us compare two strategic options for the treatment of diabetes: oral insulin vs. artificial pancreas based on biomedical technology. The critical observation from this deep analytics is that oral insulin is a rational, simple, practically feasible and safe option as compared to artificial pancreas. But in case of pancreatic cancer, artificial pancreas may be an essential option. However, the concept of artificial pancreas is a very complex and costly strategic option and there is threat of immunity and safety from the perspectives of adaptability of human biological system. But, both the aforesaid options are not matured at their technology life-cycle; they are now at emergence phase. It is also essential to adopt a set of proactive and reactive measures to fight against diabetes such as herbal and homeopathic medicines, yoga, meditation, healthy life-style, obesity control and organ replacement.
Diabetes is a disorder of deficiency in insulin, a peptide hormone of pancreas. Insulin is generally given by subcutaneous (SC) route; the non-compliance of diabetic patients is a common occurence. Oral insulin is an alternative option but it is essential to identify appropriate delivery mechanism. Oral insulin is the dream of diabetic patients. Nanotechnology may be an innovative strategic option in this connection due to the size of particles in nano range and greater surface area [3,4]. These physical and chemical properties improve the absorption of nanoparticles as compared to larger carriers. This is a real challenge of today’s research on oral insulin from the academic and industrial community. Is it possible to use nanoparticles as a carrier to deliver insulin orally?
Let us analyze the strength and opportunities of oral insulin delivery mechanism which support a cost effective, convenient, simple and painless treatment; it reduces the risk of hypoglycemic incidents, immune responses and obesity. The threat and weaknesses of SC route mechanism may be considered from various aspects such as hyperinsulinemia, lipoatrophy, lipohypertrophy, patient noncompliance, painful procedure of injections and cost for the treatment for hyperglycemia, retinopathy, neuropathy and nephropathy. There are various types of Diabetes Mellitus such as Type I, Type II, gestational and secondary.
Now, let us consider the strength of nanomedicines. For oral insulin delivery, it is possible to explore various types of options such as nanoparticles (NPs), liposomes, microemulsions (MEs), self-nanoemulsifying drug delivery systems (SNEDDS), micelles, nanogels (NGs), microspheres, niosomes, and superporous hydrogels (SPHs). A NP is a small entity, particle size ranges from 10 to 1000 nm. Two major pathways by which NPs pass through intestinal epithelium are paracellular and transcellular. Transcellular route is the most common route of absorption. NPs can be classified into polymeric and lipid-based systems. Biocompatible and biodegradable polymeric NPs may be an ideal carrier for delivering proteins and peptides orally. It improves bioavailability of oral insulin. It may be Nanospheres and nanocapsules. Solid lipid nanoparticles (SLNs) offer some advantages like nano size range and comparatively narrow size distribution, controlled release of