4.1. Buckyballs: Their history and discovery*
This module was developed as part of a Rice University Class called "Nanotechnology: Content and Context" initially funded by the National Science Foundation under Grant No. EEC-0407237. It was conceived, researched, written and edited by students in the Fall 2005 version of the class, and reviewed by participating professors.
““This year's Nobel Prize in Chemistry has
implications for all the natural sciences. The seeds of the
discovery were sowed by a desire to understand the behavior of
carbon in red giant stars and interstellar gas clouds. The
discovery of fullerenes has expanded our knowledge and changed our
thinking in chemistry and physics. It has given us new hypotheses
on the occurrence of carbon in the universe. It has also led us to
discover small quantities of fullerenes in geological formations.
Fullerenes are probably present in much larger amounts on earth
than previously believed. It has been shown that most sooty flames
contain small quantities of fullerenes. Think of this the next time
you light a candle!””
-From the presentation speech for the Nobel
Prize in Chemistry, 1996
In 1996, the Royal Swedish Academy of
Sciences awarded the Nobel Prize in Chemistry, the most prestigious
award in the world for chemists, to Richard Smalley, Robert Curl,
and Harold Kroto for their discovery of fullerenes. They discovered
fullerenes (also called buckyballs) in 1985, but the special
properties of the buckyballs took a few years to prove and
categorize. Although by 1996 no practical applications of
buckyballs had been produced, scientists appreciated the direction
this discovery based in organic chemistry had led scientific
research, as well as its specific contributions to various other
fields. The accidental discovery of fullerenes also emphasizes the
benefits and unexpected results which can arise when scientists
with different backgrounds and research aims collaborate in the
laboratory.
Before going into detail about the actual
buckyball, we should discuss the element that makes its structure
possible, carbon. Carbon is the sixth element on the periodic
table, and has been found to be at least a partial constituent in
over 90 per cent of all chemicals known to man. Indeed, its
electron-bonding properties grant it a versatility specific to
carbon, allowing it to be so widely functionalized, and more
importantly, the reason for life on Earth. Anything that is living
is necessarily chemically based on Carbon atoms, and for this
reason, substances containing carbon are called organic compounds,
and the study of them is called organic chemistry.
Though carbon is involved in chemistry with
all sorts of other elements and compounds, it can also exist in
pure carbon states such as graphite and diamond. Graphite and
diamond are two different allotropes of carbon. An allotrope is a
specific physical arrangement of atoms of an element. So although
diamond and graphite are both pure carbon, because the crystalline
structure of each is significantly different, their chemical and
physical properties (as well as value) are very different.
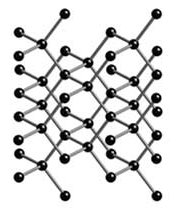
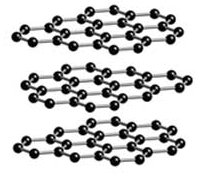
Above: diamond Below: carbon. Notice how the
structure of the two allotropes vary, even though they are both
made of the same carbon atoms (black)
Diamond and graphite are not the only known
allotropes pf carbon, chaoit and carbon(VI), discovered in 1968 and
1972, respectively, have also been found. Even more recently, the
Buckminsterfullerenes, the subject of this module, were discovered
at Rice by Smalley, Kroto,and Curl. Buckminsterfullerenes is
actually a class of allotropes
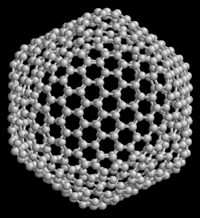
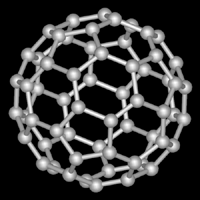
Above: C540 Below: C60 Both of these are
different allotropes of carbon. C60 is the most common and the most
popularized of the Buckminsterfullerenes. Not shown is the second
most common Buckyball, C70.
In fact, scientists have now discovered
hundreds of buckyballs of different sizes, all with the trademark
spherical-like shape. To differentiate them, each allotrope is
denoted as C (for carbon) with the number of carbon atoms in the
subscript (i.e. C80). Technically, the geometrical shapes that
these buckyballs share are actually known as geodesics, or rather,
polyhedrons that approximate spheres. Specifically, the commonly
depicted C60 buckyball is a truncated icosahedron. A more
satisfactory representation of it can be had in a soccer ball, with
which it shares the exact same shape. It is made up of 12
pentagons, each surrounded by 5 hexagons (20 in all).
British chemist Harold W. Kroto at the
University of Sussex was studying strange chains of carbon atoms
found in space through microwave spectroscopy, a science that
studies the absorption spectra of stellar particles billions of
kilometers away to identify what compounds are found in space. This
is possible because every element radiates a specific frequency of
light that is unique to that element, which can observed using
radiotelescopes. The elements can then be identified because a
fundamental rule of matter stating that the intrinsic properties of
elements apply throughout the universe, which means that the
elements will emit the same frequency regardless of where they are
found in the universe. Kroto took spectroscopic readings near
carbon-rich red giants, or old stars with very large radii and
relatively low surface temperatures, and compared them to spectrum
lines of well-characterized substances. He identified the dust to
be made of long alternating chains of carbon and nitrogen atoms
known as cynopolyynes, which are also found in interstellar clouds.
However Kroto believed that the chains were formed in the stellar
atmospheres of red giants and not in interstellar clouds, but he
had to study the particles more closely.
At the same time, Richard Smalley was doing
research on cluster chemistry, at Rice University in Houston,
Texas. “Clusters” are aggregates of atoms or molecules, between
microscopic and macroscopic sizes, that exist briefly. Smalley had
been studying clusters of metal atoms with the help of Robert Curl,
using an apparatus Smalley had in his laboratory. This
laser-supersonic cluster beam apparatus had the ability to vaporize
nearly any known material into plasma using a laser, which is a
highly concentrated beam of light with extremely high
energy.
Through an acquaintance with Curl, Kroto
contacted Smalley and discussed the possibility of using his
apparatus to recreate the high-heat conditions of a red giant’s
atmosphere in order to study the clusters of carbon produced, which
might give Kroto insight as to the formation of the carbon chains.
Smalley conceded and Kroto arrived in Smalley’s laboratory in Rice
University on September 1, 1985 whom began working on the
experiment along with graduate students J.R. Heath and S.C.
O’Brien.
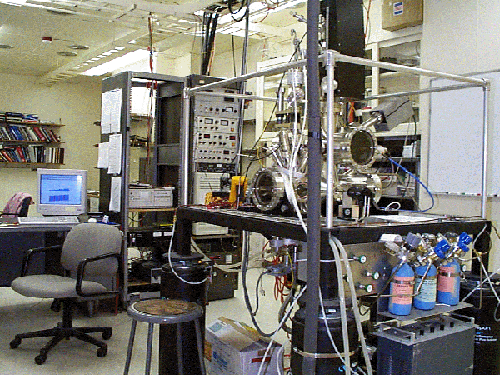
Smalley’s apparatus, shown above, fires a
high energy laser beam at a rotating disk of graphite in a
helium-filled vacuum chamber. Helium is used because it is an inert
gas and therefore does not react with the gaseous carbon. The
intense heating of the surface of the graphite breaks the C—C bonds
because of the intense energy. Once vaporized, the carbon atoms
cool and condense in the high-pressure helium gas, colliding and
forming new bond arrangements. Immediately upon cooling several
degrees above absolute zero in a chamber, the carbon leads to a
mass spectrometer for further analysis.
A mass spectrometer uses an atom or
molecule’s weight and electric charge to separate it from other
molecules. This is done by ionizing the molecules, which is done by
bombarding the molecules with high energy electrons which then
knocks off electrons. If an electron is removed from an otherwise
neutral molecule, then the molecule becomes a positively charged
ion or cation. The charged particles are then accelerated by
passing through electric plates and then filtered through a slit. A
stream of charged particles exits the slit and is then deflected by
a magnetic field into a curved path. Because all the particles have
a charge of +1, the magnetic field exerts the same amount of force
on them, however, the more massive ions are deflected less, and
thus a separation occurs. By adjusting the strength of the
accelerating electric plates or the deflecting magnetic field, a
specific mass can be selected to enter the receptor on the end.
After adjusting the experiment, it became greatly evident that the
most dominant molecule measured was 720 amu (atomic mass units). By
dividing this number by the mass of a single carbon atom (12 amu),
it was deduced that the molecule was comprised of 60 carbon atoms
(720 / 12 = 60).
The next task was to develop a model for the
structure of C60, this new allotrope of carbon. Because it was
overwhelmingly dominant, Smalley reasoned the molecule had to be
the very stable. The preferred geometry for stable molecule would
reasonably be spherical, because this would mean that all bonding
capabilities for carbon would be satisfied. If it were a chain or
sheet like graphite, the carbon atoms could still bond at the ends,
but if it were circular all ends would meet. Another hint as to the
arrangement of the molecule was that there must be a high degree of
symmetry for a molecule as stable as C60. Constructing a model that
satisfied these requirements was fairly difficult and the group of
scientists experimented with several models before coming to a
conclusion. As a last resort, Smalley made a paper model by cutting
out paper pentagons and hexagons in which he tried to stick them
together so that the figure had 60 vertices. Smalley found that he
create a sphere made out of 12 pentagons interlocking 20 hexagons
to make a ball. The ball even bounced. To ensure that the shape
fulfilled the bonding capabilities of carbon, Kroto and Curl added
sticky labels to represent double bonds. The resulting shape is
that of a truncated icosahedron, the same as that of a soccer ball.
Smalley, Curl, and Kroto named the molecule buckminsterfullerene
after the American architect and engineer Richard Buckminster
Fuller who used hexagons and pentagons for the basic design of his
geodesic domes.
Eleven days after they had begun, the
scientist submitted their discovery to the prestigious journal
Nature in a manuscript titled “C60 Buckminsterfullerene.” The
journal received it on the 13th of September and published it on
the 14th of November 1985. The controversial discovery sparked
approval and criticism for a molecule that was remarkably
symmetrical and stable.
Experimentally, Smalley, Kroto, and Curl,
first created the buckyballs using Smalley’s laser-supersonic
cluster beam apparatus to knock carbons off of a plate and into a
high pressure stream of helium atoms. They would be carried off and
immediately be cooled to only a few degrees above absolute zero,
where they would aggregate and form these buckyballs. This method
however, resulted in low yields of buckyballs, and it took nearly
five years until in 1990 newer methods developed by American and
German scientists could manufacture buckyballs in large
quantities.
The common method today involves transmitting
a large current between two graphite electrodes in an inert
atmosphere, such as Helium. This gives rise to a carbon plasma arc
bridging the two electors, which cools instantaneously and leaves
behind a sooty residue from which the buckyballs can be
extracted.
These methods of producing buckyballs do
deserve a great deal of applaud. However, humans cannot take all,
or even most of, the credit for the production of fullerenes. As a
matter of fact, buckyballs occur in nature, naturally, and in
greater amounts than expected. Buckyballs are known to exist in
interstellar dust and in geological formations on Earth. Even
closer to home are the buckyballs that naturally form in the wax
and soot from a burning candle, as the flame on the wick provides
the sufficient conditions for such processes to occur. Buckyballs
are the new sensation for us, but to Nature, they are old
news.
Chemical and Physical Properties
Since buckyballs are still relatively new,
there properties are still being heavily studied. Buckyballs’
unique shape and electron bonding give them interesting properties
on the physical level, and on the chemical level.
Since spheres in nature are known to be the
most stable configurations, one could expect the same from
fullerenes. Indeed this is one of the reasons why Smalley, Curl,
and Kroto initially considered its shape. Their tests showed that
it was extremely stable, and thus, they reasoned, it could be a
spherical-like geodesic. Also, fullerenes are resilient to impact
and deformation. This means, that squeezing a buckyball and then
releasing it would result in its popping back in shape. Or perhaps,
if it was thrown against an object it would bounce back; ironically
just like the very soccer ball it resembles.
Buckyballs are also extremely stable in the
chemical sense. Since all the carbon-carbon bonds are optimized in
their configuration, they become very inert, and are not as prone
to reactions as other carbon molecules. What makes these bonds
special is a property called aromaticity. Normally, electrons are
fixed in whatever bond they constitute. Whereas in aromatic
molecules, of which hexagonal carbon rings are a prime example,
electrons are free to move (“delocalize”) among other bonds. Since
all the fullerenes have the cyclo-hexanes in abundance, they are
very aromatic, and thus have very stable, inert, carbon bonds.
Buckyballs, though sparingly soluble in many solvents, are in fact
the only known carbon allotropes to be soluble.
An interesting feature of Fullerenes is that
their hollow structure allows them to hold other atoms inside them.
The applications of this are abound, and are being studied to great
extent.
Important to note about any new material is
its health concerns. Although believed to be relatively inert,
experiments by Eva Oberdörster at Southern Methodist University,
presented some possible dangers of fullerenes. She introduced
buckyballs into water at concentrations of 0.5 parts per million,
and found that largemouth bass suffered a 17-fold increase in
cellular damage in the brain tissue after 48 hours. The damage was
of the type lipid peroxidation, which is known to impair the
functioning of cell membranes. Their livers were also inflamed and
genes responsible for producing repair enzymes were activated. As
of 10/20/05, the SMU work had not been peer reviewed.
What have buckyballs contributed to
science?
After the astrophysicists D.R. Huffmann and
W. Kratschmer managed to produce larger quantities of fullerenes in
1990, scientists further investigated the structure and
characteristics of buckyballs. Research on buckyballs has led to
the synthesis of over 1000 new compounds with exciting properties,
and over 100 patents related to buckyballs have been filed in the
US. In addition, an important new material, nanotubes, has exploded
onto the scientific scene in recent years. The discovery and
manufacture of nanotubes resulted directly from research on
buckyballs. Finally, although buckyballs have not yet been used in
any practical applications, partly due to the high cost of
material, researchers are using buckyballs to learn more about the
history of our world, and companies are devising some interesting
uses for buckyballs even today.
The discovery of nanotubes in 1991 by S. Iijima has been
by far the buckyball’s most significant contribution to current
research. Nanotubes, both single- and multi-walled, can be thought
of as sheets of graphite rolled into cylinders and sometimes capped
with half-fullerenes. Nanotubes, like fullerenes, possess some very
unique properties, such as high electrical and thermal
conductivity, high mechanical strength, and high surface area. In
fact, carbon nanotubes provide a clear example of the special
properties inherent at the quantum level because they can act as
either semi-conductors or metals, unlike macroscopic quantities of
carbon molecules. These properties make nanotubes extremely
interesting to researchers and companies, who are already
developing many potentially revolutionary uses for them.
What are buckyballs teaching us about our
world?
A paper published on
March 28, 2000 in the Proceedings of the National Academy of
Sciences (PNAS) by Becker, Poreda, and Bunch uses buckyballs to
provide new evidence for early periods in earth’s geological and
biological history. By exploiting the unique properties of
buckyballs, these three scientists were able to study geology in a
new way. First of all, the unique ability to extract fullerenes
(unlike graphite and diamond) from organic solvents allowed them to
isolate carbon material in the meteorites, then the unique
cage-like structure of fullerenes allowed them to investigate the
noble gases enclosed within the ancient fullerenes. In their study,
the researches found helium of extraterrestrial origin trapped
inside buckyballs extracted from two meteorites and sedimentary
clay layers from 2 billion and 65 million years ago respectively.
The helium inside these buckyballs bears unusual ratios of 3He/4He
coupled with non-atmospheric ratios of 40Ar/36Ar, which according
to their research indicates extraterrestrial origin. In addition,
they have shown that these fullerenes could not have been formed
upon impact of the meteorite or during subsequent forest
fires.iBecker, Poreda, and Bunch. 2982.
The discovery of the extraterrestrial origin
of the enclosed helium has far-reaching implications for the
history of the earth. For example, the existence of the carrier
phase of fullerenes suggests that “fullerenes, volatiles, and
perhaps other organic compounds were being exogenously delivered to
the early Earth and other planets throughout time.”iiBecker,
Poreda, and Bunch, 2982. With more research, it might even be
possible to determine whether meteorite impacts on earth could have
triggered global changes or even brought carbon and gases to earth
that allowed for the development of life!
Why does it matter? Why should anyone care?
These buckyballs are giving scientists information about allotropes
of carbon never before conceived. More importantly, these
buckyballs might allow engineers and doctors do what was never
before possible. These are some of the applications for buckyballs
currently in research.
Medical uses for buckyballs
Drug Treatments
Buckyballs are now being considered for uses in the field
of medicine, both as diagnostic tools and drug candidates. Simon
Friedman, a researcher at the University of Kansas, began
experimenting with buckyballs as possible drug treatments in 1991.
Because buckyballs have a rigid structure (unlike benzene rings,
often used for similar purposes), researchers are able to attach
other molecules to it in specific configurations to create precise
interactions with a target molecule. For example, Friedman has
created a protease inhibitor that attaches to the active site of
HIV 50 times better than other molecules. C Sixty, a Toronto based
company that specializes in medical uses of fullerenes, plans to
test on humans two new fullerene-based drugs for Lou Gehrig’s
disease and HIV in the near future.
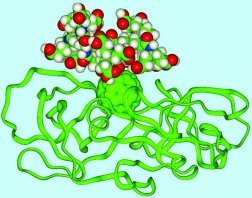
Gadolinium Carriers
Another medical use for buckyballs is taking
place in the field of diagnostics. Buckyballs unique cage-like
structure might allow it to take the place of other molecules in
shuttling toxic metal substances through the human body during MRI
scans. Usually, the metal gadolinium is attached to another
molecule and sent into the body to provide contrast on the MRI
scans, but unfortunately these molecules are excreted from the
system quickly to reduce the chance of toxic poisoning in the
subject. Lon Wilson of Rice University and researchers at TDA
Research have encased gadolinium inside buckyballs, where they
cannot do harm to the patient, allowing them to remain inside the
body longer, but still appear in MRI’s. So far this application has
been successfully tested in one rat. Wilson and others have begun
to develop even more applications for the tiny little cages that
could one day help revolutionize
medicine.
Engineering Uses
Nano STM
The Scanning Tunneling Microscope (STM) is one of the foremost tools in microscopy today; boasting the ability to to map out the topology of material surfaces at atomic resolution (i.e. on the order of 0.2 nanometers). The STM achieves this feat by bringing a needle point, functioning as a probe, within just several nanometers of a sample's surface. At these minute scales, even small disturbances can cause the tip to crach into the sample and deform itself. A possible solution to this problem would be the replacement of the standard needle point with a buckyball. As discussed previously, fullerenes bear amazing resilience due to their spherical geometry, and would resist distortions from such collisions.
Buckyballs in circuits
European scientists are aiming to use
buckyballs in circuit. So far, they have been able to attach a
single fullerene to a copper surface, and then, through a process
called shrink wrapping, fitted its center with a metal ion and made
it smaller to increases electric conductivity by a hundred
times.
Lubricants
Because of their shapes, they could be used
equivalently to ball bearings, and thus allow surfaces to roll over
each other, making the fullerenes equivalently lubricants
Superconductors
It has been shown that fitting a potassium
ion in the buckyball causes it to become superconductive. Ways to
exploit this are in the research stages.
Catalysts
Attaching metals onto the surface of
fullerenes offers the possibility for buckyballs to become
catalysts.
As we can see, we have come along way since
that fateful year of 1985. Strides have been made. We have seen the
rise of nanotubes and the new science of Nanotechnology. We are
still studying the chemical and physical properties of buckyballs
and continue to be amazed. They have already proved to us why they
are important; their possible uses in medicine and in engineering
are broad and profound, while the health risks they posed have yet
to be fully analyzed. Only time will tell whether they will meet,
or exceed our expectations as we unfold this brave new
world.
Nobelprize.org:
http://nobelprize.org/chemistry/laureates/1996/press.html
http://www.science.org.au/nova/024/024print.htm
http://blogcritics.org/archives/2004/04/10/084049.php
http://www.science.org.au/nova/024/024key.htm
http://www.sciencedaily.com//releases/2003/04/030418081522.htm
http://www.sciencenews.org/articles/20020713/bob10.asp
Gorman, Jessica. Buckymedicine: Coming soon
to a pharmacy near you?. Science News Online: July 13, 2002, vol.
162, no. 2.
http://www.sciencenews.org/articles/2002071