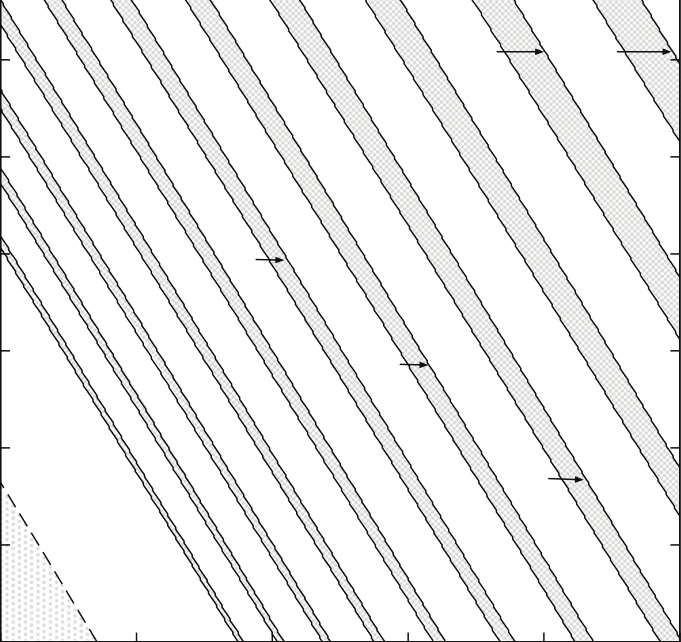
Fig. 7. Different types of oscillatory responses observed in symmetric two-coupled system at
kr = 0.89 and φ = 0.8
are inappropriate for dynamically segmenting an image with two image regions (Fujimoto
et al., 2009b).
Next,
we investigated the bifurcations of out-of-phase periodic points on the
( kr , φ )-plane. (Musashi et al., 2009). As shown in Fig. 8(b), their tangent bifurcations
and D-type of branchings were found. For example, there are stable out-of-phase m -periodic
points in the shaded parameter region surrounded by Gm and Dm
1 for m = 30, 32, 34, 36 for
the observed periodic points. Note that the overlapping parameter region indicates that
Discrete-Time Dynamic Image-Segmentation System
415
1
36
G 1
34
28
G
34
G
G 2
1
1
0.9
36
D
1
30
30
38
D
G
G
1
1
1
−→
0.8
−→
φ
φ
32
D
32
G
1
1
0.7
34
D
1
0.6
0.85
0.86
0.87
0.88
0.89
kr −→
kr −→
(a) In-phase periodic points
(b) Out-of-phase periodic points
Fig. 8. Bifurcations of perodic points observed in symmetric two-coupled system
20
20
→
→
10
10
0
0
y i
y 2
-10
+ -10
+
-20
-20
x i
x 2
0
20
40
60
80
100
-20 -10 0 10 20
t −→
x 1 + y 1 →
Fig. 9. Out-of-phase oscillatory response observed in asymmetric two-coupled system at
kr = 0.85 and φ = 0.8
out-of-phase periodic points coexist. The whole parameter region where there are stable
out-of-phase periodic points is much wider than that of stable in-phase periodic points.
This is favorable for dynamic image segmentation, because an in-phase periodic point is
unsuitable and an out-of-phase periodic point is suitable.
We set d 1 = d 2 in the two-coupled system, and therefore, the symmetry for the exchange of
( x 1, y 1) and ( x 2, y 2) in Eq. (6) is lost. This asymmetric two-coupled system corresponds to a
situation where an input image contains two image regions of different colors. No symmetric
periodic points occur in this system; however, we could observe the asymmetric out-of-phase
periodic point shown in Fig. 9. Note that it is difficult to determine whether a periodic point is
symmetric only from waveforms and phase portraits; however, this is not important because
the feasibility of dynamic image segmentation is not dependent on whether there is symmetry
or not but on the number of phases in a periodic point.
Figure 10(a) shows bifurcation sets of out-of-phase periodic points observed at d 1 = 2 and
d 2 = 1.9. Different from the symmetric system, D-type of branching never appeared due to
the asymmetric system; instead, period-doubling bifurcations were found. Comparing the
extent of all the shaded parameter regions in Figs. 8(b) and 10(a), the asymmetric system is
as wide as the symmetric system. Moreover, we set d 1 = 2 and φ = 0.8 and investigated
their bifurcations on the ( kr , d 2)-plane as seen in Fig. 10(b). This indicates that there were
stable out-of-phase periodic points even if the value of |d 1 − d 2 | was large; in other words, the
416
Discrete Time Systems
difference between the gray levels of the pixels in the two image regions is large. This is also
favorable for a dynamic image segmentation system.
1
37
0.9
I 1
28
I
38
1
1
G
32
32
35
29
29
1
G
I 1
I
35
1
2
G
1
G
2
G
0.8
36
33
33
I 2
−→
−→
28
30
I 1
1
G
1
G
1
G
36
φ
30
I
1
d 2
2
G
34
0.7
31
I
31
1
G
1
3
G
34
2
G
35
1
G
0.6
0.85
0.86
0.87
0.88
0.89
kr −→
kr −→
(a) Case of d 1 = 2 and d 2 = 1.9
(b) Case of d 1 = 2 and φ = 0.8
Fig. 10. Bifurcations of out-of-phase perodic points observed in asymmetric two-coupled
system
3.3.3 Three-coupled system
This model is composed of a global inhibitor and three neurons without direct coupling to
the others and was derived as a reduced model of our dynamic segmentation of an image
containing three image regions. As well as the aforementioned reduced models, we drew
several two-parameter bifurcation diagrams to find the parameter regions such that a stable
fixed point or a stable m -periodic point existed.
When we set d 1 = d 2 = d 3 = 2, the three-coupled system was symmetric for a
circular exchange of ( xi , yi ) for ( xi +1, yi +1), i = 1, 2, 3 where the value of i + 1 returns
to 1 if i = 3.
In this symmetric system, we found a stable fixed point, x ∗
(32.244, − 29.167, 32.244, − 29.167, 32.244, − 29.167, 0.222), at kr = 0.88 and φ = 0.8. In
the results we investigated, we found the bifurcation diagram on the fixed point on the
( kr , φ )-plane was the same as the one in Fig. 5. Moreover, as well as those in the symmetric
two-coupled system, we could observe in-phase oscillatory responses in only the right hand
side region of NS 11. The waveform of an in-phase oscillatory response and its phase portraits
are shown in Fig. 11(a), where the blue, red, and green points correspond to the responses of
the first, second, and third neurons. The results suggest that the Neimark-Sacker bifurcation
set, NS 11, causes in-phase oscillatory responses to generate and these are similar to those of the
symmetric two-coupled system (Fujimoto et al., 2009b; Musashi et al., 2009). Therefore, this
implies that the global bifurcation structure of a fixed point and the generation of in-phase
oscillatory responses are intrinsic properties of the symmetric Q -coupled system.
We also observed several oscillatory responses in certain parameter regions. Figures 11(b)
and 11(c) show a two-phase and a three-phase periodic points. For the following reasons, we
only
focused on the bifurcations of three-phase periodic points that were appropriate for
dynamically segmenting an image with three image regions.
Figure 13 shows bifurcation sets of three-phase periodic points observed in the symmetric
system. Tangent, period-doubling, and Neimark-Sacker bifurcations were observed. The
Discrete-Time Dynamic Image-Segmentation System
417
20
20
20
20
→
→
→
→
10
10
10
10
0
0
0
0
y i
y 2
y 3
y 1
-10
+ -10
-10
-10
+
+
+
-20
-20
-20
-20
x i
x 2
x 3
x 1
0
20
40
60
80
100
-20 -10 0 10 20
-20 -10 0 10 20
-20 -10 0 10 20
t −→
x 1 + y 1 →
x 2 + y 2 →
x 3 + y 3 →
(a) In-phase oscillatory response
20
20
20
20
→
→
→
→ 10
10
10
10
0
0
0
0
y i
y 2
y 3
y 1
-10
-10
+ -10
-10
+
+
+
-20
-20
-20
-20
x i
x 2
x 3
x 1
-20 -10 0 10 20
0
20
40
60
80
100
-20 -10 0 10 20
-20 -10 0 10 20
t −→
x 1 + y 1 →
x 2 + y 2 →
x 3 + y 3 →
(b) Two-phase oscillatory response
20
20
20
20
→
→
→
→ 10
10
10
10
0
0
0
0
y i
y 2
y 3
y 1
-10
-10
+ -10
+
+ -10
+
-20
-20
-20
-20
x i
x 2
x 3
x 1
-20 -10 0 10 20
0
20
40
60
80
100
-20 -10 0 10 20
-20 -10 0 10 20
t −→
x 1 + y 1 →
x 2 + y 2 →
x 3 + y 3 →
(c) Three-phase oscillatory response
Fig. 11. Different types of oscillatory responses observed in symmetric three-coupled system
at d 1 = d 2 = d 3 = 2, kr = 0.89, and φ = 0.8
20
20
20
20
→
→
→
→ 10
10
10
10
0
0
0
0
y i
y 2
y 3
y 1
-10
-10
+ -10
-10
+
+
+
-20
-20
-20
-20
x i
x 2
x 3
x 1
-20 -10 0 10 20
0
20
40
60
80
100
-20 -10 0 10 20
-20 -10 0 10 20
t −→
x 1 + y 1 →
x 2 + y 2 →
x 3 + y 3 →
Fig. 12. Three-phase oscillatory response observed in asymmetric three-coupled system at
d 1 = 2, d 2 = 1.9, d 3 = 1.8, kr = 0.89, and φ = 0.8
respective periodic points are symmetrical for the aforementioned circular exchange.
However, as seen in Fig. 13, we could find no D-type of branching in these investigations.
There is a stable three-phase periodic point in each shaded parameter region. Compared
with the extent of the entire shaded parameter region in Fig. 8(b), that of the three-phase
periodic points is small; however, it is sufficient to design the parameters of our dynamic
image segmentation system.
Next, we set d 1 = d 2 = d 3, i.e., this model is asymmetric. Although this three-coupled
system loses symmetry, there is a three-phase periodic point in certain parameters as shown in
Fig. 12. We investigated the bifurcations of several three-phase periodic points observed in the
asymmetric system and drew two bifurcation diagrams. Figure 14(a) shows the bifurcation
sets of three-phase periodic points on the ( kr , φ )-plane. Of course, we found no D-type of
Page 1 Page 2 Page 3 Page 4 Page 5 Page 6 Page 7 Page 8 Page 9 Page 10 Page 11 Page 12 Page 13 Page 14 Page 15 Page 16 Page 17 Page 18 Page 19 Page 20 Page 21 Page 22 Page 23 Page 24 Page 25 Page 26 Page 27 Page 28 Page 29 Page 30 Page 31 Page 32 Page 33 Page 34 Page 35 Page 36 Page 37 Page 38 Page 39 Page 40 Page 41 Page 42 Page 43 Page 44 Page 45 Page 46 Page 47 Page 48 Page 49 Page 50 Page 51 Page 52 Page 53 Page 54 Page 55 Page 56 Page 57 Page 58