PART
3
AD RESEARCH:
Better
Questions,
New
Answers

Scientists have studied AD from many drug treatments. Findings from current research angles. They have looked at populations are pointing scientists in promising directions for to see how many cases of AD occur
the future. They are also helping researchers to
every year and whether there might
ask better questions about the issues that are still
be links between the disease and lifestyles or
unclear.
genetic backgrounds. They also have conducted
Part 3 of Unraveling the Mystery describes what
clinical studies with healthy older people and
scientists are learning from their search for:
those at various stages of AD. They have done
many studies with laboratory animals. They
■ The causes of AD
have begun to look at neuronal circuits and
■ New techniques to help in diagnosis
networks of cells to learn how AD pathology
■ New treatments
develops and spreads. They even have examined
Results from this research will bring us closer
individual nerve cells to see how beta-amyloid, to the day when we will be able to delay the onset tau, and other molecules affect the ability of
of, prevent, or cure the devastating disease that
cells to function normally.
robs our older relatives and friends of their most
These studies have led to a fuller under-
precious possession—their minds.
standing of many aspects of the disease, improved
diagnostic tests, new ways to manage behavioral
aspects of AD, and a growing number of possible
ALZHEIMER’S DISEASE Unraveling the Mystery 35

P A R T 3 AD Research: Better Questions, New Answers Looking
for the Causes ofAD
person’s risk, such as the age at which the disease
begins. Slow and careful detective work by scientists
has paid off in discoveries of genetic links to the two
One of the most important parts of
unraveling the AD mystery is
finding out what causes the disease.
What makes the disease process begin
main types of AD.
in the first place? What makes it worse over time?
One type is the rare, early-onset Alzheimer’s
Why does the number of people with the disease
disease. It usually affects people aged 30 to 60.
increase with age? Why does one person develop
Some cases of early-onset disease are inherited and
AD while another remains healthy?
are called familial AD (FAD). The other is
Some diseases, such as measles or pneumonia,
late-onset Alzheimer’s disease. It is by far the
have clear-cut causes. They can be prevented with
more common form and occurs in those 60 and
vaccines or cured with antibiotics. Others, such as
older. Gaining insight into the genetic factors
diabetes or arthritis, develop when genetic, lifestyle, associated with both forms of AD is important and environmental factors work together to start
because identifying genes that either cause the
a disease process. The role that any or all of these
disease or influence a person’s risk of developing it
factors play may be different for each individual.
improves our ability to understand how and why
AD fits into the second group of diseases.
the disease starts and progresses.
We do not yet fully understand what causes AD,
but we believe it develops because of a complex
series of events that take place in the brain over a
long period of time. Many studies are exploring
the factors involved in the cause and develop-
ment of AD.
GENETIC FACTORS AT WORK IN AD
Genetic studies of complex neurodegenera-
tive diseases such as AD focus on two main
issues—whether a gene might influence
a person’s overall risk of developing
a disease and whether a gene might
influence some particular aspect of a

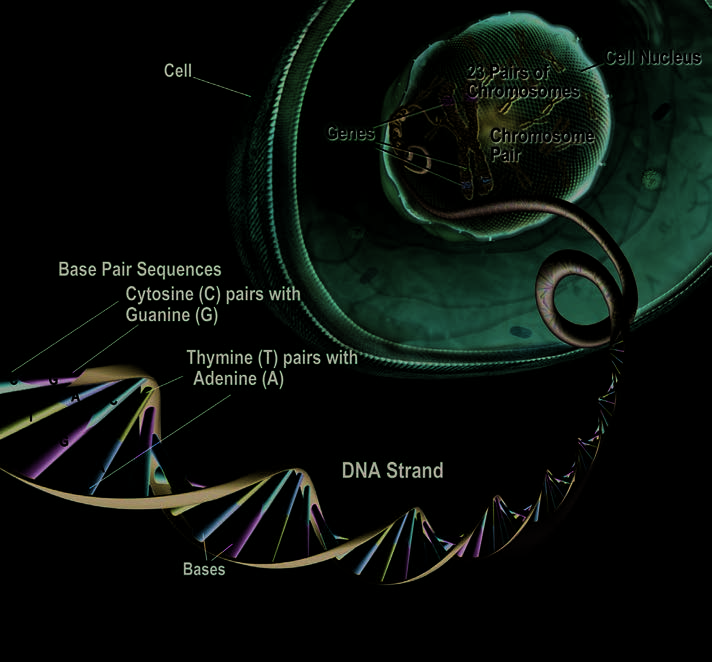
DNA, Chromosomes, and Genes: The Body’s Amazing Control Center The nucleus of almost
every human cell contains
an encrypted “blueprint,” along
with the means to decipher it. This
blueprint, accumulated over eons
of genetic trial and error, carries all
the instructions a cell needs to do
its job. The blueprint is made up of
DNA, which exists as two long,
intertwined, thread-like strands
called chromosomes. Each cell
has 46 chromosomes in 23 pairs.
The DNA in chromosomes is made
up of four chemicals, or bases,
strung together in various sequence
patterns. The DNA in nearly all
cells of an individual is identical.
Each chromosome contains
many thousands of segments,
called genes. People inherit two
copies of each gene from their
parents, except for genes on the
X and Y chromosomes, which are
construction, operation, and repair. DNA that causes a disease is chromosomes that, among other
Even though all genes are present
called a mutation. Mutations
functions, determine a person’s sex. in most cells, the pattern in which also can change the activation
Each person normally has one pair they are activated varies from cell of a particular gene. Other more
of sex chromosomes (females are
to cell, and gives each cell type
common (or frequent) changes in
XX and males are XY). The
its distinctive character. Even slight
a gene’s sequence of bases do not
sequence of bases in a gene tells
alterations in a gene can produce
automatically cause disease, but
the cell how to make specific
an abnormal protein, which, in turn, they can increase the chances that proteins. Proteins in large part deter- may lead to cell malfunction and, a person will develop a particular
mine the different kinds of cells that
eventually, to disease.
disease. When this happens,
make up an organism and direct
Any permanent change in the
the changed gene is called a
almost every aspect of the cell’s
sequence of bases in a gene’s
genetic risk factor.
ALZHEIMER’S DISEASE Unraveling the Mystery 37
P A R T 3 AD Research: Better Questions, New Answers Genes and Early-Onset
A Different Genetic Story in
Alzheimer’s Disease
Late-Onset Alzheimer’s Disease
In the early days of AD genetics research, scientists
While some scientists were studying the role of
realized that some cases, particularly of the rare
chromosomes 21, 14, and 1 in early-onset AD,
early-onset AD, ran in families. This led them to
others were looking elsewhere to see if they could
examine DNA samples from these families to see
find genetic clues for the late-onset form. By 1992,
whether they had some genetic trait in common.
investigators had narrowed their search to a region
Chromosomes 21, 14, and 1 became the focus of
of chromosome 19. They found a gene on
attention. The scientists found that some families
chromosome 19 that they were able to link to
have a mutation in selected genes on these chromo-
late-onset AD.
somes. On chromosome 21, the mutation causes an
This gene, called APOE, produces a protein
abnormal amyloid precursor protein to be produced called apolipoprotein E. APOE comes in several (see page 22 for more on APP). On chromosome
forms, or alleles—ε2, ε3, and ε4:
14, the mutation causes an abnormal protein called
presenilin 1 to be produced. On chromosome 1,
■ The APOE ε2 allele is relatively rare and may
the mutation causes another abnormal protein to be provide some protection against the disease. If AD
produced. This protein, called presenilin 2, is very
does occur in a person with this allele, it develops
similar to presenilin 1. Even if only one of these
later in life than in those with an APOE ε4 allele.
genes that are inherited from a parent contains a
■ APOE ε3 is the most common allele. Research-
mutation, the person will almost inevitably develop ers think it plays a neutral role in AD.
early-onset AD. This means that in these families,
■ APOE ε4 occurs in about 40 percent of all
children have about a 50-50 chance of developing
people who develop late-onset AD and is present
the disease if one of their parents has it.
in about 25 to 30 percent of the population. Peo-
Early-onset AD is very rare, and mutations in
ple with AD are more likely to have an APOE ε4
these three genes do not play a role in the more
allele than people who do not have AD. However,
common late-onset AD. However, these findings
at least one-third of people with AD do not have
were crucial because they showed that genetics was an APOE ε4 allele. Dozens of studies have con-indeed a factor in AD, and they helped to identify firmed that the APOE ε4 allele increases the risk some key cell pathways involved in the AD disease of developing AD, but how that happens is not process. They showed that mutations in APP can
yet understood. These studies also have helped to
cause AD, highlighting the presumed key role of
explain some of the variation in the age at which
beta-amyloid in the disease. Mutations in pre-
AD develops, as people who inherit one or two
senilin 1 and 2 also cause an increased amount of
APOE ε4 alleles tend to develop AD at an earlier
the damaging beta-amyloid to be made in the brain. age than those who do not. However, inheriting an APOE ε4 allele does not mean that a person
will definitely develop AD. Some people with one
or two APOE ε4 alleles never get the disease, and
others who do develop AD do not have any APOE
ε4 alleles.
38 ALZHEIMER’S DISEASE Unraveling the Mystery

The Hunt for New AD Genes
For some time, scientists have In 2003, NIA launched the
suspected that, in addition
Alzheimer’s Disease Genetics
to APOE ε4, as many as half a
Study to identify at least 1,000
dozen other risk-factor genes exist
families with members who have
for late-onset AD, but they have
late-onset AD as well as members
been unable to find them. In 2007, who do not have the disease. All scientists unveiled their discovery of of these family members provide one new AD risk-factor gene.
blood samples and other clinical
This AD risk-factor gene is
data for the initiative. The material
called SORL1. It is involved in
collected allows investigators to
recycling APP from the surface of
create and maintain “immortalized”
cells, and its association with AD
cell lines—cells that are continuous-
was identified and confirmed in
ly regenerated in the laboratory.
three separate studies. Researchers These cell lines are crucial for the found that when SORL1 is
exhaustive DNA analysis studies
expressed at low levels or in a
needed to identify risk-factor genes, be drawn from existing samples variant form, harmful beta-amyloid each of which may have relatively of blood and tissue; other genetic
levels increase, perhaps by
small effects on AD development.
material will be collected from new
deflecting APP away from its
More than 4,000 new cell lines
participants.
normal pathways and forcing it
are now available for researchers
New AD genetics discoveries
into cellular compartments that
to study risk-factor genes for
are possible largely because
generate beta-amyloid.
late-onset AD.
of close collaboration among
As AD genetics research has
A new initiative, the Alzheimer’s scientists, participation of volunteer intensified, it has become increas-Disease Genetics Consortium,
families, new genetics technol-
ingly clear that scientists need
was launched in 2007 to accel-
ogies, statistical and analytic
many different samples of genetic
erate the application of genetics
advances, and rapid data sharing.
material if they are to continue
technologies to late-onset AD
For example, the SORL1 studies
making progress in identifying new through collaborations among most involved 14 scientific institutions in risk-factor genes. Genetic material
of the leading researchers in AD
North America, Europe, and Asia
is also essential for identifying
genetics. The ultimate goal of this
and the participation of more than
associated environmental factors
effort is to obtain genetic material
6,000 people who donated blood
and understanding the interactions from 10,000 people with AD and and tissue for genetic typing. An of genes and the environment.
10,000 cognitively healthy people important part of NIA’s efforts to These advances ultimately will
to comprehensively scan the whole promote and accelerate AD
allow investigators to identify people genome for the remaining AD
genetics research is to make
at high risk of developing AD and
risk-factor genes, as well as those
biological samples and data
help them focus on new pathways
for age-related cognitive decline.
publicly available to approved
for prevention or treatment.
Some of the genetic material will
researchers.
ALZHEIMER’S DISEASE Unraveling the Mystery 39
P A R T 3 AD Research: Better Questions, New Answers OTHER FACTORS AT WORK IN AD
refined antibody approaches are now being tested
Genetics explains some of what might cause AD,
in clinical trials, and additional research on new
but it does not explain everything. So, researchers
ways of harnessing the antibody response contin-
continue to investigate other possibilities that may ues in the lab.
explain how the AD process starts and develops.
Another important area of research is how
beta-amyloid may disrupt cellular communication
Beta-Amyloid
well before plaques form. One recent study
We now know a great deal about how beta-
described how beta-amyloid oligomers target
amyloid is formed and the steps by which
specific synaptic connections between neurons,
beta-amyloid fragments stick together in small
causing them to deteriorate. Other scientists are
aggregates (oligomers), and then gradually form
studying other potentially toxic effects that plaques
into plaques (see page 22 in The Hallmarks of
have on neurons and in cellular communication.
AD for more on this process). Armed with this
Understanding more about these processes may
knowledge, investigators are intensely interested
allow scientists to develop specific therapies to
in the toxic effects that beta-amyloid, oligomers,
block the toxic effects.
and plaques have on neurons. This research is
possible in part because scientists have been able
Tau
to develop transgenic animal models of AD.
Tau, the chief component of neurofibrillary tangles Transgenics are animals that have been specially
(see page 25 in The Hallmarks of AD for more
bred to develop AD-like features, such as
on tau), is generating new excitement as an area
beta-amyloid plaques.
of study. The recent focus on tau has been spurred
Beta-amyloid studies have moved forward to
by the finding that a mutant form of the protein
the point that scientists are now carrying out
is responsible for one form of frontotemporal
preliminary tests in humans of potential therapies dementia, the third most common cause of late-life aimed at removing beta-amyloid, halting its
dementia, after AD and vascular dementia. This
formation, or breaking down early forms before
form is known as frontotemporal dementia with
they can become harmful.
parkinsonism linked to chromosome 17 (FTDP-
For example, one line of research by a pharma-
17). Finding this mutant protein was important
ceutical company started with the observation that because it suggested that abnormalities in the tau injecting beta-amyloid into AD transgenic mice
protein itself can cause dementia.
caused them to form antibodies to the beta-
New transgenic mouse models of AD have
amyloid and reduced the number of amyloid
helped tau research make rapid progress. For
plaques in the brain. This exciting finding led to
example, a recent model, the “triple transgenic”
other studies and ultimately to clinical trials in
mouse, forms plaques and tangles over time in
which human participants were immunized with
brain regions similar to those in human AD.
beta-amyloid. These studies had to be stopped
Another recent transgenic mouse model, which
because some of the participants developed
contains only human tau, forms clumps of
harmful side effects, but the investigators did
damaging tau filaments also in a region-specific
not give up hope. Rather, they went back to the
fashion similar to AD in humans.
drawing board to rethink their strategy. More
These studies of tau also have suggested a
mechanism for tau damage that is different from
that previously suspected. With these new insights,
40 ALZHEIMER’S DISEASE Unraveling the Mystery
scientists now speculate that one reason tau may
Protein Misfolding
damage and kill neurons is because it upsets
Researchers have found that a number of devastat-
the normal activity of the cell, in addition to
ing neurodegenerative diseases (for example, AD,
forming neurofibrillary tangles.
Parkinson’s disease, dementia with Lewy bodies,
Other studies of mutant tau in mice suggest that
frontotemporal lobar degeneration, Huntington’s
the accumulation of tau in tangles may not even be the disease, and prion diseases) share a key culprit in memory loss. Rather, as with beta-amyloid,
characteristic—protein misfolding.
it may be that an earlier and more soluble abnormal
When a protein is formed, it “folds” into a
form of the protein causes the damage to neurons.
unique three-dimensional shape that helps it
Researchers Explore Neurodegenerative “Cousins”
Neurodegenerative diseases like AD, Parkinson’s a combination of genetic, lifestyle, and environmental disease, amyotrophic lateral sclerosis (ALS),
causes and they develop over many years.
and dementia with Lewy bodies share more than the
This graphic shows one way of thinking about
basic characteristic of misfolded proteins. They also
how these diseases may be linked as well as what
share clinical characteristics. For example, people
makes them unique. By investigating the unique
with AD have trouble moving, a characteristic of
characteristics of these diseases as well as the
Parkinson’s disease. Sleep-wake disorders, delusions, characteristics they share, scientists hope to learn psychiatric disturbances, and memory loss occur in
even more than they would if they focused on each
all of these diseases. These diseases also result from
disease by itself.
Damaging Processes
Lifetime
Occurring Before
Neurodegenerative
Influences
Symptoms Appear
Early Symptoms
Diseases*
Tremor
Memory loss
Amyloid plaques
AD/PD
Executive function
AD
Tau tangles
DLB
PD
problems
Genes
Other abnormal
PDD
Movement problems
Environment
protein deposits
VaD
Gait and balance problems
Systemic
Reduced oxygen
factors
Sleep-wake disorders
flow to tissues
FTLD
Hallucinations
Toxic processes
ALS
Delusions
Rigidity
* AD = Alzheimer’s disease, AD/PD = AD with parkinsonism, ALS = amyotrophic lateral sclerosis, DLB = dementia with Lewy bodies, FTLD = frontotemporal lobar degeneration, VaD = vascular dementia (includes multi-infarct dementia), PD = Parkinson’s disease, PDD = Parkinson’s disease with dementia
Adapted from an Emory University illustration
ALZHEIMER’S DISEASE Unraveling the Mystery 41
P A R T 3 AD Research: Better Questions, New Answers perform its specific function. This crucial process
Scientists do not know exactly why or how
can go wrong for various reasons, and more
these processes occur, but research into the unique
commonly does go wrong in aging cells. As a
characteristics and actions of various misfolded
result, the protein folds into an abnormal shape—
proteins is helping investigators learn more about
it is misfolded. In AD, the misfolded proteins are
the similarities and differences across age-related
beta-amyloid (the cleaved product of APP; see
neurodegenerative diseases. This knowledge may
From APP to Beta-Amyloid Plaques on page 22
someday lead to therapies.
for more on the formation of beta-amyloid)
and a cleaved product of









