Chapter 21
Oxygen, Nitrogen, Sulphur Cycles
The OXYGEN CYCLE is the biogeochemical cycle that describes the movement of oxygen within and between its three main reservoirs: the atmosphere (air), the biosphere (living things), and the lithosphere (Earth's crust). The main driving factor of the oxygen cycle is photosynthesis, which is responsible for the modern Earth's atmosphere and life.
Reservoirs and fluxes
By far the largest reservoir of Earth's oxygen is within the silicate and oxide minerals of the crust and mantle (99.5%). Only a small portion has been released as free oxygen to the biosphere (0.01%) and atmosphere (0.36%). The main source of atmospheric oxygen is photosynthesis, which produces sugars and oxygen from carbon dioxide and water:
6CO2 + 6H2O + energy → C6H12O6 + 6O2
Photosynthesizing organisms include the plant life of the land areas as well as the phytoplankton of the oceans. The tiny marine cyanobacterium Prochlorococcus was discovered in 1986 and accounts for more than half of the photosynthesis of the open ocean.[1]
An additional source of atmospheric oxygen comes from photolysis, whereby high energy ultraviolet radiation breaks down atmospheric water and nitrite into component atoms. The free H and N atoms escape into space leaving O2 in the atmosphere:
2H2O + energy → 4H + O2
2N2O + energy → 4N + O2
The main way oxygen is lost from the atmosphere is via respiration and decay, mechanisms in which animal life and bacteria consume oxygen and release carbon dioxide.
Because lithospheric minerals are oxidised in oxygen, chemical weathering of exposed rocks also consumes oxygen. An example of surface weathering chemistry is formation of iron-oxides (rust):
4FeO + O2 → 2Fe2O3
Oxygen is also cycled between the biosphere and lithosphere. Marine organisms in the biosphere create calcium carbonate shell material (CaCO3) that is rich in oxygen. When the organism dies its shell is deposited on the shallow sea floor and buried over time to create the limestone rock of the lithosphere. Weathering processes initiated by organisms can also free oxygen from the lithosphere. Plants and animals extract nutrient minerals from rocks and release oxygen in the process. The PHOSPHORUS CYCLE is the biogeochemical cycle that describes the movement of phosphorus through the lithosphere, hydrosphere, and biosphere. Unlike many other biogeochemical cycles, the atmosphere does not play a significant role in the movements of phosphorus, because phosphorus and phosphorus-based compounds are usually solids at the typical ranges of temperature and pressure found on Earth.
Phosphorus is an essential nutrient for plants and animals in the form of ions PO43- and HPO42- . It is a part of DNA-molecules and RNA-molecules, molecules that store energy (ATP and ADP) and of fats of cell membranes. When Phosphorus is in a compound (phosphate), it plays an important role in holding the DNA and RNA together.[1] Phosphorus is also a building block of certain parts of the human and animal body, such as the bones and teeth.
Phosphorus cycle is also known as a Sedimentary cycle because the cycle does not enter the atmosphere and stays in rock sediments, sand,and ocean floor. Where it is trapped until erosion occurs and the Phosphorus is released.
Phosphorus forms parts of important life sustaining molecules but is not very common in the biosphere. Phosphorous does not enter the atmosphere remaining mostly on land and in rock and soil minerals. 80 percent of the phosphorus is used to make fertilizers and a type of phosphorus such as dilute phosphoric acid is used in soft drinks. Phosphates may be effective in such ways but they also cause pollution problems in lakes and streams. Over enrichment of phosphate can lead to algal bloom, because of the excess of nutrients. This causes more algae to grow, bacteria consumes the algae and causes more bacteria to increase in numbers. They use all the oxygen in the water during cellular respiration, causing many fish to die.
Phosphorus normally occurs in nature as part of a phosphate ion, consisting of a phosphorus atom and some number of oxygen atoms, the most abundant form (called orthophosphate) having four oxygens: PO43-. Most phosphates are found as salts in ocean sediments or in rocks. Over time, geologic processes can bring ocean sediments to land, and weathering will carry terrestrial . Plants absorb phosphates from the soil, then bind the phosphate into organic compounds. The plants may then be consumed by herbivores who in turn may be consumed by carnivores. After death, the animal or plant decays, and the phosphates are returned to the soil. Runoff may carry them back to the ocean or they may be reincorporated into rock.
The primary biological importance of phosphates is as a component of nucleotides, which serve as energy storage within cells (ATP) or when linked together, form the nucleic acids DNA and RNA. Phosphorus is also found in bones, whose strength is derived from calcium phosphate, and in phospholipids (found in all biological membranes).
Phosphates move quickly through plants and animals; however, the processes that move them through the soil or ocean are very slow, making the phosphorus cycle overall one of the slowest biogeochemical cycles.
However, recent findings suggest that phosphorus is cycled through the ocean on the timescale of 10,000 year, suggesting that the phosphorus cycle may play a role in global warming.
Plants bind the phosphate into organic compounds. (Phosphate is not very common in biospheres)Unlike other cycles of matter compounds, phosphorus cannot be found in air as a gas. This is because at normal temperature and circumstances, it is a solid in the form of red and white phosphorus. It usually cycles through water, soil and sediments. Phosphorus is typically the limiting nutrient found in streams, lakes and fresh water environments. As rocks and sediments gradually wear down, phosphate is released. In the atmosphere phosphorus is mainly small dust particles.
Phosphorus is one of the longest cycles, and takes a long time to move from sediments to living organisms and back to sediments.
Initially, phosphate weathers from rocks. The small losses in a terrestrial system caused by leaching through the action of rain are balanced in the gains from weathering rocks. In soil, phosphate is absorbed on clay surfaces and organic matter particles and becomes incorporated (immobilized). Plants dissolve ionized forms of phosphate. Herbivores obtain phosphorus by eating plants, and carnivores by eating herbivores. Herbivores and carnivores excrete phosphorus as a waste product in urine and feces. Phosphorus is released back to the soil when plants or animal matter decomposes and the cycle repeats.
SULFUR CYCLE
Sulfur is one of the constituents of many proteins, vitamins and hormones. It recycles as in other biogeochemical cycles.
The essential steps of the sulfur cycle are:
-
Mineralization of organic sulfur to the inorganic form, hydrogen sulfide: (H2S).
-
Oxidation of sulfide and elemental sulfur (S) and related compounds to sulfate (SO42–).
-
Reduction of sulfate to sulfide.
-
Microbial immobilization of the sulfur compounds and subsequent incorporation into the organic form of sulfur.
These are often termed as follows:
Assimilative sulfate reduction in which sulfate (SO42–) is reduced to organic sulfhydryl (otherwise known as thiol) groups (R–SH) by plants, fungi and various prokaryotes. The oxidation states of sulfur are +6 in sulfate and –2 in R–SH.
Desulfuration in which organic molecules containing sulfur can be desulfurated, producing hydrogen sulfide gas (H2S), oxidation state = –2. Note the similarity to deamination.
Oxidation of hydrogen sulfide produces elemental sulfur (So), oxidation state = 0. This reaction is done by the photosynthetic green and purple sulfur bacteria and some chemolithotrophs.
Further oxidation of elemental sulfur by sulfur oxidizers produces sulfate.
Dissimilative sulfur reduction in which elemental sulfur can be reduced to hydrogen sulfide.
Dissimilative sulfate reduction in which sulfate reducers generate hydrogen sulfide from sulfate.
Human impact on the sulfur cycle is primarily in the production of sulfur dioxide (SO2) from industry (e.g. burning coal) and the internal combustion engine. Sulfur dioxide can precipitate onto surfaces where it can be oxidized to sulfate in the soil (it is also toxic to some plants), reduced to sulfide in the atmosphere, or oxidized to sulfate in the atmosphere as sulfuric acid, a principal component of acid rain.
WATER CYCLE
The different processes are as follows:
-
Precipitation is condensed water vapor that falls to the Earth's surface. Most precipitation occurs as rain, but also includes snow, hail, fog drip, graupel, and sleet. Approximately 505,000 km³ of water fall as precipitation each year, 398,000 km³ of it over the oceans.
-
Canopy interception is the precipitation that is intercepted by plant foliage and eventually evaporates back to the atmosphere rather than falling to the ground.
-
Snowmelt refers to the runoff produced by melting snow.
-
Runoff includes the variety of ways by which water moves across the land. This includes both surface runoff and channel runoff. As it flows, the water may infiltrate into the ground, evaporate into the air, become stored in lakes or reservoirs, or be extracted for agricultural or other human uses.
-
Infiltration is the flow of water from the ground surface into the ground. Once infiltrated, the water becomes soil moisture or groundwater.
-
Subsurface Flow is the flow of water underground, in the vadose zone and aquifers. Subsurface water may return to the surface (eg. as a spring or by being pumped) or eventually seep into the oceans. Water returns to the land surface at lower elevation than where it infiltrated, under the force of gravity or gravity induced pressures. Groundwater tends to move slowly, and is replenished slowly, so it can remain in aquifers for thousands of years.
-
Evaporation is the transformation of water from liquid to gas phases as it moves from the ground or bodies of water into the overlying atmosphere. The source of energy for evaporation is primarily solar radiation. Evaporation often implicitly includes transpiration from plants, though together they are specifically referred to as evapotranspiration. Total annual evapotranspiration amounts to approximately 505,000 km³ of water, 434,000 km³ of which evaporates from the oceans.
-
Sublimation is the state change directly from solid water (snow or ice) to water vapor.
-
Advection is the movement of water — in solid, liquid, or vapour states — through the atmosphere. Without advection, water that evaporated over the oceans could not precipitate over land.
-
Condensation is the transformation of water vapour to liquid water droplets in the air, producing clouds and fog.
Effects on climate:The water cycle is powered from solar energy. 86% of the global evaporation occurs from the oceans, reducing their temperature by evaporative cooling. Without the cooling effect of evaporation the greenhouse effect would lead to a much higher surface temperature of 67 °C, and a warmer planet.
Effects on biogeochemical cycling
While the water cycle is itself a biogeochemical cycle, flow of water over and beneath the Earth is a key component of the cycling of other biogeochemicals. Runoff is responsible for almost all of the transport of eroded sediment and phosphorus from land to waterbodies. The salinity of the oceans is derived from erosion and transport of dissolved salts from the land. Cultural eutrophication of lakes is primarily due to phosphorus, applied in excess to agricultural fields in fertilizers, and then transported overland and down rivers. Both runoff and groundwater flow play significant roles in transporting nitrogen from the land to waterbodies. The dead zone at the outlet of the Mississippi River is a consequence of nitrates from fertilizer being carried off agricultural fields and funnelled down the river system to the Gulf of Mexico. Runoff also plays a part in the carbon cycle, again through the transport of eroded rock and soil. Human activities that alter the water cycle include:
-
agriculture
-
alteration of the chemical composition of the atmosphere
-
construction of dams
-
deforestation and afforestation
-
removal of groundwater from wells
-
water abstraction from rivers
-
urbanization
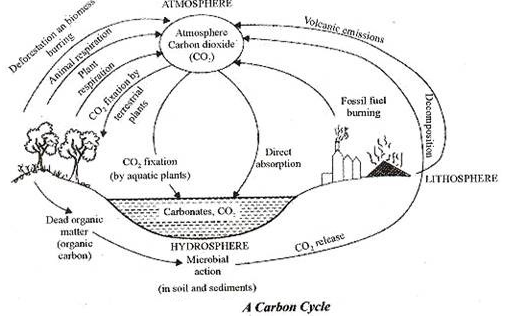
Carbon Cycle:
The carbon cycle is the biogeochemical cycle by which carbon is exchanged among the biosphere, pedosphere, geosphere, hydrosphere, and atmosphere of the Earth. It is one of the most important cycles of the earth and allows for carbon to be recycled and reused throughout the biosphere and all of its organisms.
The Carbon Cycle is a complex series of processes through which all of the carbon atoms in existence rotate. The wood burned just a few decades ago could have produced carbon dioxide which through photosynthesis became part of a plant. When you eat that plant, the same carbon from the wood which was burnt can become part of you. The carbon cycle is the great natural recycler of carbon atoms.
Without the proper functioning of the carbon cycle, every aspect of life could be changed dramatically. Plants, animals, and soil interact to make up the basic cycles of nature. In the carbon cycle, plants absorb carbon dioxide from the atmosphere and use it, combined with water they get from the soil, to make the substances they need for growth. The process of photosynthesis incorporates the carbon atoms from carbon dioxide into sugars.
Animals, such as the rabbit eat the plants and use the carbon to build their own tissues. Other animals, such as the fox, eat the rabbit and then use the carbon for their own needs. These animals return carbon dioxide into the air when they breathe, and when they die, since the carbon is returned to the soil during decomposition. The carbon atoms in soil may then be used in a new plant or small microorganisms. The following major reservoirs of carbon interconnected by pathways of exchange:
i. The atmosphere.
ii. The terrestrial biosphere, which is usually defined to include fresh water systems and non-living organic material, such as soil carbon.
iii. The oceans, including dissolved inorganic carbon and living and non-living marine biota.
iv. The sediments including fossil fuels
v. The Earth’s interior, carbon from the Earth’s mantle and crust is released to the atmosphere and hydrosphere by volcanoes and geothermal systems.he annual movements of carbon, the carbon exchanges between reservoirs, occur because of various chemical, physical, geological, and biological processes. The ocean contains the largest active pool of carbon near the surface of the Earth, but the deep ocean part of this pool does not rapidly exchange with the atmosphere in the absence of an external influence, such as an uncontrolled deep-water oil well leak.
The global carbon budget is the balance of the exchanges (incomes and losses) of carbon between the carbon reservoirs or between one specific loop the carbon cycle.
Carbon is released into the atmosphere in several ways:
i. Through the respiration performed by plants and animals. This is an exothermic reaction and it involves the breaking down of glucose (or other organic molecules) into carbon dioxide and water.
ii. Through the decay of animal and plant matter. Fungi and bacteria break down the carbon compounds in dead animals and plants and convert the carbon to carbon dioxide if oxygen is present, or methane if not.
iii. Through combustion of organic material which oxidizes the carbon it contains, producing carbon dioxide (and other things, like water vapour). Burning fossil fuels such as coal, petroleum products releases carbon dioxide. Burning agro fuels also releases carbon dioxide
iv. Volcanic eruptions and metamorphism release gases into the atmosphere. Volcanic gases are primarily water vapour, carbon dioxide and sulphur dioxide.
v. Carbon is transferred within the biosphere as heterotrophs feed on other organisms or their parts (e.g., fruits). This includes the uptake of dead organic material (detritus) by fungi and bacteria for fermentation or decay.
vi. Most carbon leaves the biosphere through respiration. When oxygen is present, aerobic respiration occurs, which releases carbon dioxide into the surrounding air or water, following the reaction C6H12O6 + 602 —> 6CO2 + 6H2O. Otherwise, anaerobic respiration occurs and releases methane into the surrounding environment, which eventually makes its way into the atmosphere or hydrosphere (e.g., as marsh gas or flatulence).
Circulation of carbon dioxide:
i. Plants absorbs the carbon dioxide from the atmosphere.
ii. During the process of photosynthesis, plants incorporates the carbon atoms from carbon dioxide into sugars.
iii. Animals, such as the rabbit eat the plants and use the carbon to build their own tissues, chain the carbon content
iv. Through the food chain, carbon is transferred into foxes, lions etc.
v. The animals return carbon dioxide into the air when they breathe, and when they die, since the carbon is returned to the soil during decomposition
In Case of Ocean:
In regions of oceanic upwelling, carbon is released to the atmosphere. Conversely, regions of down welling transfer carbon (CO2) from the atmosphere to the ocean. When CO2 enters the ocean, it participates in a series of reactions which are locally in equilibrium:
i. Conversion of CO2 (atmospheric) to CO2 (dissolved).
ii. Conversion of CO2 (dissolved) to carbonic acid (H2CO3).
iii. Conversion of carbonic acid (H2CO3) to bicarbonate ion.
iv. Conversion of bicarbonate ion to carbonate ion.
In the oceans, dissolved carbonate can combine with dissolved calcium to precipitate solid calcium carbonate, CaCO3, mostly as the shells of microscopic organisms. When these organisms die, their shells sink and accumulate on the ocean floor. Over time these carbonate sediments form limestone which is the largest reservoir of carbon in the carbon cycle.
The dissolved calcium in the oceans comes from the chemical weathering of calcium-silicate rocks, during which carbonic and other acids in groundwater react with calcium-bearing minerals liberating calcium ions to solution and leaving behind a residue of newly formed aluminium-rich clay minerals and insoluble minerals such as quartz.
The flux or absorption of carbon dioxide into the world’s oceans is influenced by the presence of widespread viruses within ocean water that infect many species of bacteria. The resulting bacterial deaths spawn a sequence of events that lead to greatly enlarged respiration of carbon dioxide, enhancing the role of the oceans as a carbon sink.
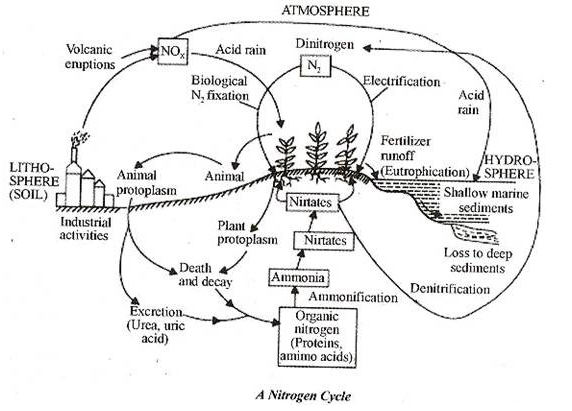
Nitrogen Cycle:
The nitrogen cycle is the set of biogeochemical processes by which nitrogen undergoes chemical reactions, changes form, and moves through difference reservoirs on earth, including living organisms.
Nitrogen is required for all organisms to live and grow because it is the essential component of DNA, RNA, and protein. However, most organisms cannot use atmospheric nitrogen, the largest reservoir. The five processes in the nitrogen cycle
i. Nitrogen fixation
ii. Nitrogen uptake
iii. Nitrogen mineralization
iv. Nitrification
v. De-nitrification
Humans influence the global nitrogen cycle primarily through the use of nitrogen-based fertilizers.
I. Nitrogen fixation: N2 -> NH4+
Nitrogen fixation is the process wherein N2 is converted to ammonium, essential because it is the only way that organisms can attain nitrogen directly from the atmosphere. Certain bacteria, for example those among the genus Rhizobium, are the only organisms that fix nitrogen through metabolic processes.
Nitrogen fixing bacteria often form symbiotic relationships with host plants. This symbiosis is well-known to occur in the legume family of plants (e.g. beans, peas, and clover). In this relationship, nitrogen fixing bacteria inhabit legume root nodules and receive carbohydrates and a favourable environment from their host plant in exchange for some of the nitrogen they fix. There are also nitrogen fixing bacteria that exist without plant hosts, known as free-living nitrogen fixers. In aquatic environments, blue-green algae (really a bacteria called cyanobacteria) is an important free-living nitrogen fixer.
II. Nitrogen uptake: NH4+ -> Organic N
The ammonia produced by nitrogen fixing bacteria is usually quickly incorporated into protein and other organic nitrogen compounds, either by a host plant, the bacteria itself, or another soil organism.
III. Nitrogen mineralization: Organic N ->NH4+
After nitrogen is incorporated into organic matter, it is often converted back into inorganic nitrogen by a process called nitrogen mineralization, otherwise known as decay. When organisms die, decomposers (such as bacteria and fungi) consume the organic matter and lead to the process of decomposition.
During this process, a significant amount of the nitrogen contained within the dead organism is converted to ammonium. Once in the form of ammonium, nitrogen is available for use by plants or for further transformation into nitrate (NO3–) through the process called nitrification
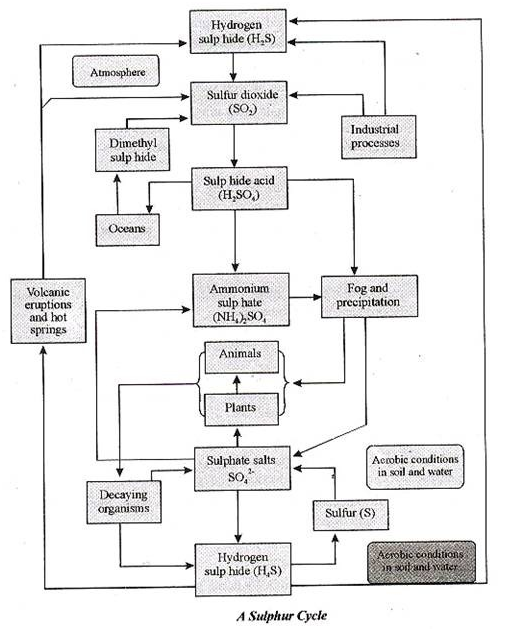
Sulphur Cycle:
Sulphur is one of the components that make up proteins and vitamins. Proteins consist of amino acids that contain sulphur atoms. Sulphur is important for the functioning of proteins and enzymes in plants, and in animals that depend upon plants for sulphur.
It enters the atmosphere through both natural and human sources. Natural recourses can be for instance volcanic eruptions, bacterial processes, evaporation from water, or decaying organisms. When sulphur enters the atmosphere through human activity, this is mainly a consequence of industrial processes where sulphur dioxide (SO2) and hydrogen sulphide (H2S) gases are emitted on a wide scale.
When sulphur dioxide enters the atmosphere it will react with oxygen to produce sulphur trioxide gas (SO3), or with other chemicals in the atmosphere, to produce sulphur salts. Sulphur dioxide may also react with water to produce sulphuric acid (H2SO4). Sulphuric acid may also be produced from demethyl-sulphide, which is emitted to the atmosphere by plankton species.
All these particles will settle back onto earth, or react with rain and fall back onto earth as acid deposition. The particles will then be absorbed by plants again and are released back into the atmosphere, so that the sulphur cycle will start over again.
i. Fossil fuels like coal and petroleum are extremely important energy resources which are getting exhausted.
ii. Hydrocarbon fuel based resources create pollution levels and green house gases. Their management is related to improved technology and finding alternative energy sources taking this into account.
iii. An overall prudent and sustainable uses of resources both at an individual and collective level can benefit a wide cross section of society as well meet the future generations.
___________________________________________________________










