Chapter 4 -
INORGANIC POLLUTANTS
Study of groundwater degradation by inorganic pollutants and various removal techniques
________________________________________________________
4A DEGRADATION OF GROUND WATER DUE TO FLOURIDE, RELATION TO FLUOROSIS IN HUMAN POPULATION AND DEFLUORIDATION TECHNOLOGIES-
The presence of disease causing micro-organisms and/or excessive dissolved compounds and salts of fluorides, nitrates,metals like iron, arsenic, lead, chromium, mercury, cadmium, copper in surface and ground water sources leads to contamination of potable drinking waters. Ground water is severely threatened by pollution due to industrial wastes and excessive inputs of population. River water pollution is mainly through industrial waste waters from paint, pigment, chrome and leather tanning, electroplating ,textile dyeing andpulp and paper industry ,cotton textile , steel industry , ceramic industries ,galvinisation of iron products , iron ore mining . Drinking water is one of the basic necessities of life. It has been observed that water is a major source of pollutants and contaminants which cause several ailments. Large number of people run the risk of suffering the adverse affects when water is unsafe to drink. WHO estimates indicate that 80% of the diseases are associated with contaminated water The common diseases are dental and skeletal fluorosis and methaemoglobinemia hepatitis A, polio, typhoid, cholera, dysentery
FLUOROSIS
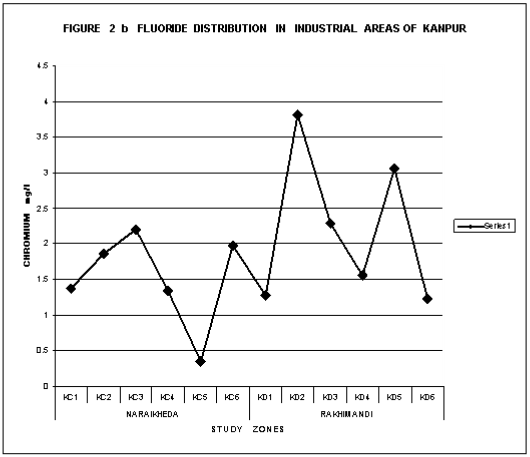
Fluorosis is a crippling disease affecting nearly 25 million population in the country. Fluorosis problem is wide spread all over the country the endemic states are Andhara Pradesh, Karnataka, Tamil Nadu, Uttar Pradesh, Haryana, Punjab, Maharastra, Gujarat, Rajasthan, Jammu Kashmir, Delhi, Kerala. Table 1 give a summarized information on the occurrence of fluoride in ground water in some districts in different states of India. The fluoride distribution in the ground water of various towns of Rajasthan are shown in Figure 1&2 ,where fluoride exceeds 1.5 mg\l permissible limit .

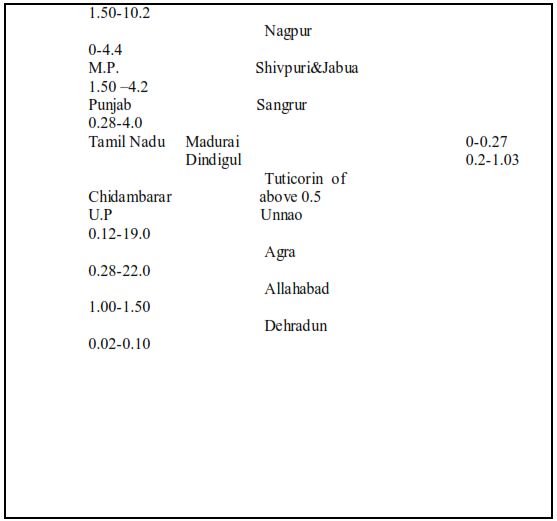
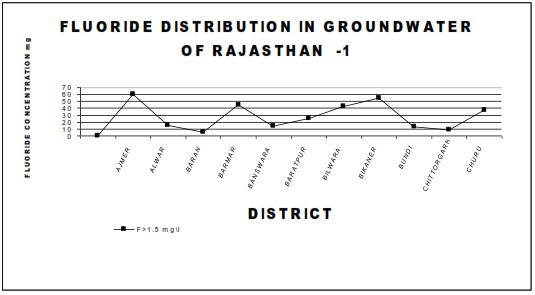
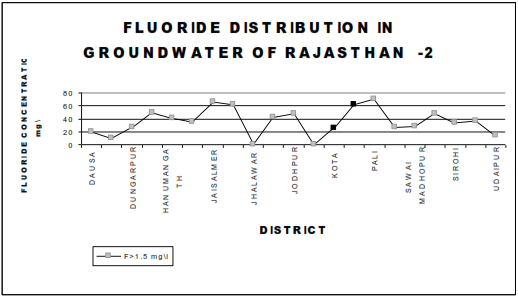
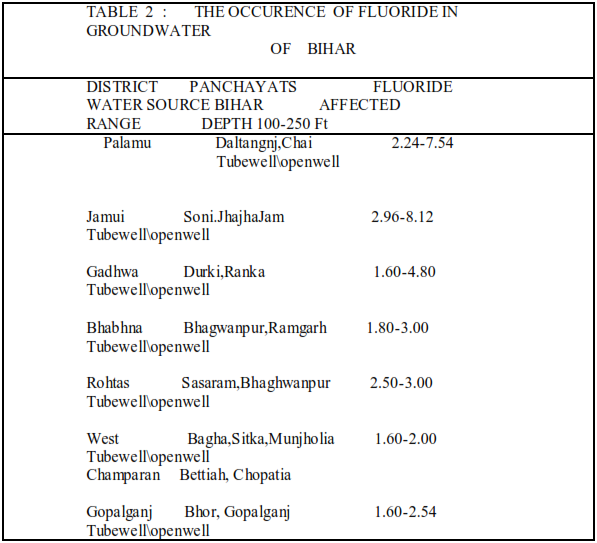
The fluoride problem in Bihar is acute where around 30% districts have excess fluoride in ground water as per survey , these districts are Palamu ,Jamui, Gadhwa,
Bhabhna, Rohtas, West Champaran, Gopalganj .Due to high fluoride concentration in water almost all children in the affected villages are suffering from dental fluorosis and a large number of adults are suffering from skeletal fluorosis .[Table 2]
FLOURIDE RELEASING INDUSTRIES
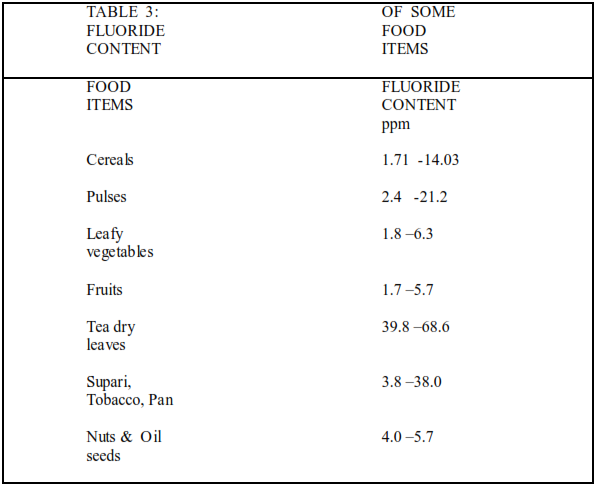
Large scale of industrial growth has caused serious concern regarding the suscepility of ground water due to heavy metals . Waste materials near the factories are subjected to reaction with percolating rain water and reaches the groundwater level . The permissible limit for fluoride in drinking water 1.5mg\l [.Industries using hydrofluoric acid or fluoride salts in their technology i.e.aluminium industries,steel and enamel, pottery and glass industries,oil refineries , pharmaceuticals and cosmetic industries which liberate flourous gases to the atmosphere and are the main cause of industral induced Fluorosis.Certain beverages like dry tea leaves contain 39.8 –68.6 ppm fluoride Chewing items like supari ,tobacco,pan contain 3.8-38.0 ppm fluoride . Use of fluoride bearing toothpaste and mouth wash lead to Fluorosis .[Table 3 ]
RELATION OF HIGH FLUORIDE INTAKE TO FLUOROSIS
There are. two types of fluorosis ,dental and skeletal fluorosis Dental fluorosis occurs when the level of fluoride in water exceeds 1.5 mg/l. Teeth due to their high calcium content easily take up fluoride. Prolonged intake of water having fluoride levels exceeding 3 mg/l leads to skeletal fluorosis leads to bow legs and knock knees which affects children as well as adults symptoms of this disease tingling knees. sensation in the legs followed by pain, stiffness of the back.
SYMPTOMS OF SKELETAL FLUOROSIS
In patients showing effects of skeletal fluorosis ,the medical reports suggest accumulation of fluoride ion more in cancellous bones than in cortical bone ,because of porosity ,copious blood supply,presence of trabecular bone surfaces and bone engulfing bone marrow
The fluorosed bone shows characteristic structural changes viz.
*increased bone mass and density.
*exostosis( bone outgrowth) at bone surfaces
*increased osteoid seam and resorption surfaces.
*increased trabecular bone volume,cortical porosity and periosteocytic lacunar surface.
*formation of unminneralizid cartilaginous loci within the trabeculae of the cacellous bone
*maximum ill effects are detected in the neck spine knee pelvic and shoulder joints and . small joints of hands and feet
*severity of skeletal flurosis ,increases pain which is associated with rigidity and restricted movement of cervical and lumber spine ,knee,pelvic and shoulder joints .
*further severity results stiff spine i.e. Bamboo spine , immobile knee ,pelvic and shoulder joints.
*cripplingdeformityfurther results :Kyphosis,Scoliosis,Hexion deformity,Paraplegia.
Quadriplegia and Osteophytes
MECHANISM OF FLUORIDE ACCUMULATION IN THE SOIL
Fluoride in lithosphere and soil found chemically in compound forms i.e. flouraphite ,fluorite and other rock forming minerals in order of 0.06% -0.09% of the earth crust . Salt deposists of marine orgin also contribute fluoride to the soil. The fluoride accumulation in soil is governed by (i) natural solubility of fluoride compounds (ii) acidity of the soil (iii) presence of other minerals & chemical compounds (iv) amount of water present . The fluoride accumulation in soil is also depth factor .
ACCUMULATION OF FLUORIDE IN THE HUMAN BODY
The accumulation of fluoride in the human body is due to the high reactivity of fluoride ion with calcium of teeth bones ,resulting to form calcium flourphosphate (florapatite) crystals and leaving unbound calcium in certain locations in the same tissue,which gets calcified and in turn results in stiffness of tissues & joints causing skeletal fluorosis in late stages .the chemical substitution of fluoride ions replacing hydroxl ions and calcium hydroxy - apatite in bones is due to strong affinity between fluoride and biological apatite of the body .once the apatite/fluorhydrxyapatite forms ,it remains chemically stable until the tissue is reabsorbed or metabolized .A little of fluoride increase in the body by diffusion and absorbtion .Nearly 90% of fluoride in the body is associated with calcified tissues . The fluoride effects on proteins and on DNA molecules are also possible
DEFLUORIDATION METHODS
It has been observed that, there is a pressing need for development and introduction of simple and inexpensive methods of water treatment. Water Treatment technologies and purification methods are being tested, several pilot projects have been set up by Department of Science and Technology in rural Rajasthan . Inexpensive defluoridation units have been tested in laboratory and field. application .
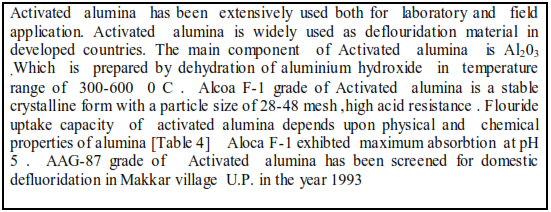
Ion exchange , Nalgonda technology,and Absorption on activated alumina have been reviewed in this light . Fishbone charcoal has been used for fluoride removal Alum treated fly ash as low cost adsorbent as deflouridation material
Ion exchange is such a process ,that removes unwanted ions from raw water by trans ferring them to a solid material called an ion-exchanger ,which accepts while giving back an equivalent number of a desirable species stored on the ion-exchanger skeleton.
The ion –exchanger has a limited capacity for storage of ions on its skeleton called its ion-exchange capacity ,because of this the ion-exchanger eventually depleted of its desirable ions and saturated with unwanted ions. It is then washed with a strong ,regenerating solution containing desirable ions ,and then these replace the accumulated undesirable ions,returning the ion-exchanger to usable condition.[Patterson et al 1988] This operation is a cyclic chemical process.Ion Exchange technology is a defluoridation method where 30,000 litres\day can be treated, the contact bed consists of a strong base anion exchange resin with an exchange capacity for fluoride ions filled in a pressure vessel.The water from the bore well is pumped into the pressure vessel and the water treated is collected in an overhead tank.Ion Exchange defluoridation unit was installed at Methsana village near Sidhpur town in Gujarat in 1997 In Table 4 Nalgonda technology and Ion Exchange technology have been compared .
Nalgonda technology is a chemical pretreatment method for defluoridation of well waters , electicity is required to operate this technology and the treatment capacity of water is about 10,000litres\day. Water is stored in tank and alum and lime are added for precipitation of dissolved impurities , followed by flocculation and sedimentation \ filteration , later treated with bleaching powder . Water treated by Nalgonda technology contains residual aluminium in the range of 2.1 - 6. 8 mg\ l under various operating conditions . Al in concentration of 80 μ g \ l or more results in Dialysis dementia and risk of Alzheimer’ s disease .
A domestic water filter was fitted with alumina casket for defluoridation of drinking water in the kitchen.[Karthikeyan,G et al 1994] This deflouridation unit is a modified model of the original house filter containing two chambers , a detachable alumina casket in the place of the silica candle in the upper chamber below it , is the water collecting chamber. Total cost of the defluoridation unit including activated alumina cost Rs 650 = 00 and the frequency of regeneration is three months .
UNICEF sponsored the development of domestic and hand pump attachable defluoridation units using activated alumina. The study area for field performance was Unnao district near Kanpur city inU.P
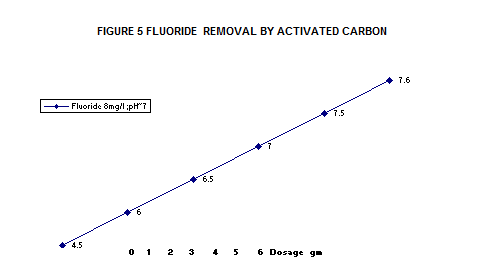
Some low cost adsrbents have been tested for fluoride removal , results have shown in
Figure 3 Aloe vera , Figure 4 water hyscinth root , Figure 5 activated carbon
1
CONCLUSION
Based upon the above literature survey the following conclusion can be drawn
(i) factors responsible for spread and severity of fluorosis
*population over growth ,consumption of more water for daily use
*unplanned and indiscrimination in digging of wells
*increase in rate of industrialization
*high level of fluoride intake through drinking water and fluoiride bearing food stuff like tea and chewing items supari , tobacco, pan .
*unawareness towards fluorosis
(ii) Several defluoridation technologies have been studied and the process of absorption on alumina has an edge other methods due to its simplicity and sludge free operation. Activated alumina has been widely recommended as a defluoridating material in developed countries.
(iii ) Alum based defluoridation technologies should be avoided It has been observed that, there is a pressing need for development and introduction of simple and inexpensive methods of water treatment.
REFERENCES
Dr Jyotsna Lal, Degradation of ground water due to flouride, relation to fluorosis in human population and defluoridation technologies- Indian . J. Environmental Protection 21,753-757 [2001]
Dr Jyotsna Lal, Toxic effect of high fluoride intake J Ecophysiology occupational health 2, [ 2002 ]
Chapter 4
4BTOXIC EFFECT OF MERCURY
Mercury is known to be one of the most toxic metals. Many areas in the world are contaminated by small-scale gold mining and industrial use of mercury, constituting serious environmental problems. Removal of mercury from industrial wastewater has been achieved by means of ion-exchangeable resin or other chemical processes. However,such chemical processes are generally expensive and sensitive to environmental conditions, and they require enormous quantities of chemicals. Therefore, new cost-effective, non-sensitive and sustainable technologies for the removal of mercury are needed
DEVELOPMENT OF A BIOLOGICAL MERCURY REMOVAL SYSTEM
.Several studies on processes for biological mercury removal mainly investigated sorption, accumulation and reduction. In particular, mercuric reduction processes are more efficient because that can transform highly toxic water-soluble ionic mercury to insoluble metallic mercury. It is important that active bacterial cells are maintained at high concentration during mercuric reduction processes. In present study, the removal of mercuric chloride by immobilized cells of mercury-volatilizing bacterium,Pseudomonas putida PpY101/pSR134 was examined The mercury removal activity of bacterial cells immobilized on various carriers was investigated..
Materials and methods
A genetically engineered mercury-volatilizing bacterium, P. putida PpY101/pSR134 was used throughout this study. Removal of mercuric chloride was examined by immobilized cells of genetically modified Pseudomonas putida PpY101/pSR134 which was endowed with mercury volatilizing activity. The immobilized cells on calcium alginate exhibited the highest mercury volatilization activity in various carriers. Immobilized cells have highly stability of mercury removal activity against high temperatures and storage than free cells, and maintained mercury removal activity after four times of removal experiments. The experiment using the mercury removal-reactor system demonstrated that about only 80-85% of the added mercury was recovered in the Hg-trapping solution while about 95% of added mercury was removed from reaction mixture. Electron micrographs of the immobilized beads and electron dispersive x-ray spectroscopy (EDS) analyses showed that part of the volatilized mercury was entrapped in gel matrix of immobilized beads.
Removal of mercuric chloride by various immobilized cells.
The calcium alginate immobilized cells and free cells exhibited the almost same mercury removal rate at 5 and 10 mg/L of mercuric chloride. The free cells removed almost of 20 mg/L of mercuric chloride for 1 hr, while about 75% and 40% of added mercury were removed by calcium alginate and strontium alginate immobilized cells. Mercury removal rates of the cells immobilized by agar and the photo-cross-linkable resin prepolymer were lower than those of calcium alginate and strontium alginate immobilized cells. Thefree cells and immobilized cells could remove mercury up to 100 mg/L. There was no removal or sorption of mercury by the gel beads without cells. . Immobilization on calcium alginate demonstrated the most effective removal rate in all the carriers. However, the mercury removal rates and the affinity for mercury of free cells were higher than those of all immobilized cells. These results indicated that immobilization decreased the mercury removal rate and affinity for mercury of this mercury-volatilizing bacterium.
Removal of mercuric chloride by immobilized cells using a mercury removal-recovery system.Removal of mercuric chloride by calcium alginate and strontium alginate immobilized cells was examined using a mercury removal-recovery system can collect bacterial volatilized-mercury. Free cells removed 20 mg/L of mercuric chloride after 2 hrs . Calcium alginate and strontium alginate immobilized cells removed all mercuric chloride after 6 hrs, however, there was no mercury removal in the control flask without cells.Almost all of the added mercury was recovered in the mercury-trapping solution by free cells. Although about 95% of the added mercury was removed from the flasks, only approximately 85% and 80% of the added mercury were recovered by calcium alginate and strontium alginate immobilized cells, respectively Residual Hg (%)
ANAEROBIC/AEROBIC BIOREMEDIATION OF CHLORINATED ORGANIC- AND MERCURY-POLLUTED SITES
Pesticides (DDT), poly-chlorinated benzenes (CBs) and heavy metals such as As and Hg are common contaminants responsible for the environmental toxicity and health hazard of petrochemical industrial sites.
Many chlorinated hydrocarbons are considered in the US EPA priority list of hazardous pollutants . The commercially available soil remediation technologies are thermal desorption of chlorinated aromatics, pesticides and Mercury and hydraulic ligant stabilization or soil washing of heavy metals other than Hg. A not invasive and low-impacting (environmentally friendly) process technology for achieving CBS and DDT biodegradation (dechlorination) coupled with metal inertization (metal sulfides precipitation) is based on the metabolic diversity of specific soil microbial communities (sulfate-reducing bacteria, SRB) and individual populations (dehalorespiratory bacteria, DB). This process involves a first step of reductive biotransformation under anaerobic sulfidogenic conditions with sulfates, chlorinated aromatics and pesticides as terminal electron acceptors Anaerobic dehalogenating processes show encouraging activity levels towards higher halogenated aromatics (and aliphatics) (Drzyzga et al., 2001). For instance, DDT can be reductively dechlorinated to DDD (2,2’ - Bis (p-chlorophenyl)- 1,1-dichloroethane) and dehydrochlorinated to DDE (2,2’ - Bis (p-chlorophenyl)- 1,1-dichloroethylene) . Recently, further dechlorination of DDE and dehydrochlorination of DDD to DDMU (1-chloro-2,2-bis (p-chlorophenyl) ethylene) mechanisms have beenproved in both methanogenic and sulfidogenic marine sediments The biogenic SRB-mediated sulfide salts precipitation results in the stabilization of metals in less exchangeable and less toxic insoluble form Due to both halo-aromatics dechlorination and metal sulfides generation the toxicity of the treated soil decreases markedly . Studies have been conducted on the use of indigenousmicrobes for the bio-detoxification of xenobiotics in sequential anaerobic-aerobic systems . These studies demonstrated a faster degradation of dechlorinated compounds under subsequent aerobic oxidizing conditions.Little research has been performed on the remediation and detoxification of heavy metals/xenobiotic-containing soils. Our purpose was to study at a lab-scale the sequential process of the degradation/ detoxification of inorganic and organic soil pollutants based on autochthonous subsurface environment anaerobic and aerobic microflora with a low cost electron donor.
In this work a soil contaminated with toxic and persistent chlorinated aromatic hydrocarbons (DDT (1,1,1-trichloro-2-2-bis(p-chlorophenyl)ethane), chlorinated benzenes (CBs)) and heavy metals, (mainly Hg and As) has been studied at the laboratory scale. In particular, a process consisting of a sequential anaerobic-aerobic soil treatment has been investigated. Experiments with soil samples highly contaminated with DDT isomers (2600 mg/kg dwb) and based on enrichment of indigenous anaerobic soil microflora with discontinuous addition of nutrient (electron donor) showed good dechlorination levels of DDT and CBs. At the same time, a sizeable decrease of the exchangeable/available Hg and As fractions, and a corresponding increase of more stable metal species (sulfides and silicates) were obtained. This resulted in a strong reduction of the whole soil toxicity (solid phase Microtox TM toxicity assay). Further degradation of halo-aromatic intermediates occurred in the aerobic step where natural heterotrophs have been stimulated by sparging air into the soil. The results confirmed the technical feasibility of the sequential bioremediation process. According to the results of a preliminary technical-economical assessment, the process shows favourable economical perspectives (estimated costs 78 Euro/t).
Site background
Soil samples collected from a 250000 m 2 petrochemical site located in a valley in northern Italy were used. Since the early twenties the plant was used for the production of a variety of chemicals such as chlorine, explosives, sulfuric acid from pyrite, ammonia, carbon tetrachloride, chlorinated aromatics and pesticides (DDT). The plant is located on alluvial, permeable soil consisting of gravel and sand with a depth of 40 m below groundsurface (bgs). The water table is located at a depth fluctuating between 5 and 7 m bgs.Environmental data from a previous general site characterization estimated an average soil DDT concentration of 250 mg/kg dwb.
Conclusions
The technical feasibility of the sequential process for the petrochemical polluted soil bioremediation by stimulating matrix autochthonous anaerobic (SRB and DB trophic groups) and aerobic (heterotrophic) specific microbiota was demonstrated at lab scale. Efficient dehalogenation of poly-chlorinated hydrocarbons (DDT and CBs) and heavy metal (Hg and As) inertization as sulfides were accomplished by anaerobic treatment of the soil as submerged and flooded slurry. The effectiveness of the anaerobic treatment were confirmed by a decrease of the whole soil toxicity. The subsequent aerobictreatment yielded further degradation of most residual halo-aromatics in mixed anaerobic/anoxic/aerobic conditions. According to preliminary economical evaluations this on site applicable biotechnology results cost effective in comparison to available .
REMEDIATION OF MERCURY-CONTAMINATED SLUDGE AND MINE WASTE USING SILICA MICRO ENCAPSULATION
Mercury (Hg) is a classical poison, with a complex chemistry that often makes remedial treatment of contaminated solids technically difficult and expensive. Consequently, containment or landfilling of wastes remains dominant. However, these are increasingly out of step with the concepts of sustainable development. KEECO has developed the Silica Micro Encapsulation (SME) process as an alternative approach. Hg-contaminated sludge from a chloralkali plant waste lagoon in British Columbia (containing 2,250 _g g -1 total recoverable Hg) and mine waste from Sulfur Bank Mercury Mine (a Superfund site) (containing _ 2,000 _g g -1 total Hg) were successfully treated during independently verified studies using the SME process. Both in situ and ex situ treatment approaches were assessed using column studies and field trials and data demonstrated that encapsulation of these contaminated wastes significantly reduced the concentration of leachable Hg (up to 75% reduction for the sludge and 88% for the mine waste). Operating costs per metric ton treated were estimated at $15.00 for the sludge, and $18.26 for the mine waste). In the case of the mine waste study, technical and economic performance of the SME process exceeded that of the two competing approaches being assessed (sulfide and phosphate-based treatments)
Silica micro encapsulation
The focus of this paper is the treatment of Hg-contaminated wastes using the SME process. Results from the treatment of two waste types are reported: contaminated sludge (from a lagoon at a former chloralkali facility in British Columbia, Canada – CASE STUDY A) and a contaminated mine waste (from the Sulfur Bank Hg Mine Superfund site – CASE STUDY B).
SME is a technology specifically developed for the removal and stabilization of heavy metals in contaminated liquids and solids. The process has been subjected to close regulatory, technical and economic scrutiny in North America and Europe by regulators, private and publicly owned corporations,
NGOs and other potential stakeholders. It has consistently demonstrated its ability to meet stringent regulatory requirements at a price competitive with established and alternative technologies. The process is also capable of treating ‘difficult’ metals such as chromium, arsenic, Hg, molybdenum and selenium in a wide range of liquid and solid materials, including waters, soils and industrial residues. These are ‘difficult’ metals because there are few effective, economic alternatives to ‘dig and dump’. SME is unique in that ituses silica to minimize the bioavailability and toxicity of heavy metals in waters, soils, sediments and other solid and liquid phases.
Column study methodology.
Two samples of waste material were collected from the SBMM site for testing; one served as the primary test material and the other as secondary. This paper focuses only on results from the primary test sample, labeled ‘white material’. In addition to KB-SEA TM , the test included the use of a proprietary inorganic
sulfide treatment chemical and a generic phosphate treatment. Four-kilogram sub-samples of the raw material were loaded into 3-foot long, 3” diameter PVC columns and treated with the vendor-supplied chemical reagents. Low flow leaching was performed for twelve weeks with weekly sampling for leachable Hg and other metal contaminants. KEECO performed thetreatment using both in situ chemical application and an ex situ application. KB-SEA TM slurry was prepared by mixing the dry chemical with deionized water. For the ex situ column samples, the chemical slurry was evenly broadcast over the test material and mixed by hand turning. For the in situ column samples, the untreated materials were loaded into the columns prior to chemical amendment. A pre-determined volume of chemical slurry was then applied to each column and allowed to infiltrate through the pore spaces. In addition to the kinetic column leach testing, a series of secondary tests were also conducted. They included the Synthetic Precipitation Leaching Procedure (SPLP), humidity cell testing and humic/fulvic acid leaching as well as SEM/EDM and XRD analysis to evaluate mineralogical changes arising from treatment (full results of these tests have been published elsewhere (EPA, 2001)). In addition to the technical evaluations, each technology vendor was also asked to conceptualize a treatment system for full-scale technology application and provide cost estimates for treatment based on the laboratory-scale data.
Column study results and conclusions.
Testing conducted on untreated samples indicated that leachable Hg was primarily associated with particulates and was correlated with turbidity. Therefore, the remedial solution would require a technology that can minimize the release of particulates as well as dissolved Hg from the material. For purposes of these tests, mobile Hg was defined as the Hg associated with the <25_m filtered fraction. The success of each treatment was measured versus untreated control columns and each column was prepared in triplicate. Each technology was evaluated for the reduction in the total mass of Hg leached from the columns as compared to the untreated control columns with a target goal of achieving 90% reduction.
REFERENCES
Paper L-11, in: V.S. Magar and M.E. Kelley (Eds.), In Situ and On-Site Bioremediation—2003.
Proceedings of the Seventh International In Situ and On-Site Bioremediation Symposium (Orlando, FL;June 2003). ISBN 1-57477-139-6, published by
Battelle Press, Columbus, OH, www.battelle.org/bookstore.
Kazuhhiro Iwassaki (kiwasaki@nies.go.jp) (National Institute for Environmental Studies,
Tsukuba, Ibaraki, Japan) Shohei Okino (Research Institute of Innovative Technology for the Earth, Kyoto, Japan),
Osami Yagi (The University of Tokyo, Bunkyo-Ku, Tokyo, Japan)Hideo Tanaka (University of Tsukuba, Tsukuba, Ibaraki, Japan)
Paper 2H-10, in: A.R. Gavaskar and A.S.C. Chen (Eds.), Remediation of Chlorinated and RecalcitrantCompounds—2002.Amy Anderson (aanderson@keeco.com) and Marcee Cameron
(Klean Earth Environmental Company, Lynnwood, WA, USA)
Dr Paul Mitchell (Environmental Consultant, PO Box 54, Camborne, UK)
Proceedings of the Third International Conference on Remediation of Chlorinated and Recalcitrant
Compounds (Monterey,CA; May 2002). ISBN 1-57477-132-9, published by Battelle Press, Columbus, OH,www.battelle.org/bookstore.
M. Camilli, R. Sisto, and E. D'Addario (EniTecnologie S.p.A., Monterotondo, Rome,
Italy) A. Bernardi, G. Franzosi (Polimeri Europa, Ist. G. Donegani, Novara, Italy)
Chapter 4c
REMEDIATION OF LEADBYLOW COST ADSORBENTS
Lead is a toxic heavy metal with cumulative effects Figure 1 shows the presence of lead in the ground water of Kanpur city [1] The presence of lead in drinking water above the permissible level ( 0.05mg/l ) causes various types of diseases anemia and CNS syndrome also gastric disorders, kidney and liver problems , changes in endocrine glands like thyroid and adrenal glands. Lead is deposited on the bones as a cumulative poison and is stored in teeth ,soft tissues including brain. The main anthropogenic pathway through which lead enters into the aquatic environment is via waste waters from industrial process such as storage batteries, paper and pulp, lead smelting ,mining, petroleum refinery, ammunition , ceramic and glass industries. Lead is also used in storage batteries, insecticides, food,beverages,ointments and medicinal concoction for flavouring and sweetening . Lead accumulation in the













