Chapter 6
Biosorption
BIOSORPTION—A VIABLE TECHNOLOGY
It has long been known that many biological materials will accumulate metals This phenomenon tends to exacerberate the environmentalimpact of various metals that are released inthe environment because they become concentrated in the flora and \or fauna and ultimately get into the food chain . How ever , the same phenomena potentially can be used in a controlled environment to remove dissolved metal from processing streams either for recovery of valuable resources or to remove pollutants . The interaction between microbes and metals has been studied by scientists of different disciplines. Agricultural scientists are primarily interested in the microbicidal action of metals , bio inorganic chemists basically look at the metal coordinating geometry and their environment in metallo proteins andbiological functionsScientists in life sciences study the toxicological effects ,its bio accumulation andbio magnification while the environmental scientists and engineers try to utilize this property of bio accumulation for monitoring metal pollution as well as removal \ recovery of metals from natural and waste waters . There is an increasing interest in investigating suchbiological adsorbents for variety of applications , as biologically derived material can be produced at low cost , yet have adsorbtion capacities near those of commercial ion-exchange resins . Adsorbtion of the metal ions on to materials of biological origin is now regarded as one ofthe most promising technologies for treating wastes economically . In this paper the author wishes to direct attention towards the application of micro organisms for metal reclaimation and recovery It has been observed that many microbial species exhibit metal binding properties the adsorption capacities of microbial biomass are comparable to or can exceed those of inorganic adsorbents . The use of bio adsorbents .for removal and recovery of dissolved metals has been demonstrated on alaboratory scale or with small pilot plants 1,2 Biosorptionhas emerged as a viable alternative to conventional metal scavenging techniques . To make this technology economically viable, however the dynamics of this reaction must be enhanced significantly Some mechanisms have been proposed by reseachers for metal accumulation by micro organisms
BIO SORPTION BY MICROBIALBIOMASS
Use of yeast cells Saccharomyces sp for starch fermentation in ethanol production is well knownResearch in use of mixed culture of Saccharomyces diastaticus and Zymomonas mobilis in ethanol production has been reported 3Biosorption , is the process in which live or dead microbes are used for removal of metals from their dilute solutions In recent years investigation of various types of micro organisms for metal adsorption has been reported Heavy metals , which can be toxic to living organisms are present in many industrial aqueous waste streams . These metals may originate from a variety of sources including the nuclear power defence and fuel reprocessing industries ; surface finishing processes in the aerospace industry ; and silk –screening processes in the computer industry. In addition , ground waters at many sites are contaminated with these metals ions making their removal andconcentration a neccessary step in the restoration of these areas.Large scale industrial growth has caused serious concern regarding the suscepility of ground water due to heavy metals , waste materials near the factories are subjected to reaction withpercolating rain water and reaches the ground water level 4 There are a number of toxic trace elements found in natural andwaste waters , some of these are essential at low levels of concentrations serving as nutrients for animals and plants but toxic at higher levels . 5 Zn essential in many metallo-enzymes toxic to plants at high levels , Cu essential trace element ,non to animals but toxic to plants and algae while As ,Hg Pb Cd are highly toxic even at low concentrations . These metals attack the active sites of enzymes , inhibiting essential enzyme activity . Heavy metals act as effective enzymes inhibitors . Zn (II) in some metallo - enzymes is substituted by Cd (II) .
As (III) attacks -SH groups of anenzyme i.e inactivation of pyruvate dehydrogenase by complexation with As (III) preventing the genaration of ATP in the citric acid cycle . As (III) ion causes arsenicosis , widely prevalent in regions of rural West Bengal . Pb inhibits sevaral key enzymes involved in process of heme synthesisin humansHg came into the limelight with the incidence ofthe Minamata disease
Many microbial species exhibit metal binding propertiesMost research in this area has been associated with heavy metals , such asPb, Cd, Cu , Cr ,and radio nuclide accumulation. Intra cellular accumulation of many metals has been observed in bacteria , fungi and algae . It has been shown[Table 1 ] by several researchers 7that for some types of microbial biomass , most of the metal adsorption occurs in the cell ormembrane wall ,this included microbial biomass from fungi such as Ganoderma lucidumwhich adsorbs Cu 17 , bacteria such asMicrococcus luteus for adsorption of Sr 18and yeastSaccharomyces cerevisae for U , Zn , Mg , Co 19Bioaccumulation of Ni, Cu bynon pathogenicyeast Candica sp 23 Copper was accumulated by Pseudomonas sp 34Artificial ponds and streams contain filamentatous algae and cyanobacteria cangreatly reduce levels Pb ,Cu , Zn and Mn from waste waters
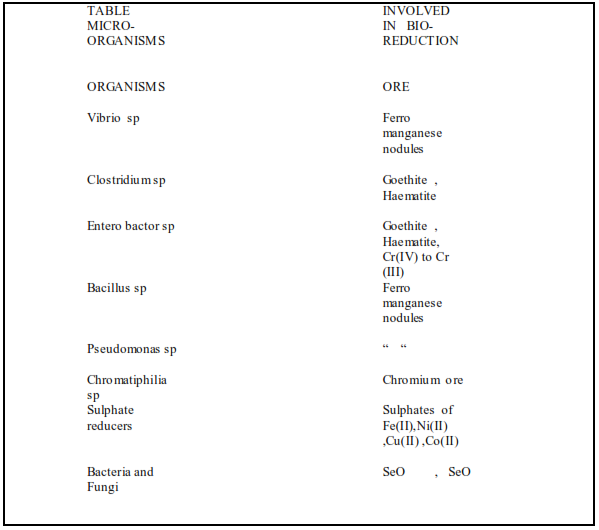
The live cyano bacteria Anabena cylindrica , Anabena flos-aquae and Nostoc spaccumulatesNi 11 Arthrobacterhas high Ni uptake 12 Chlorella vulgaris has affinity for Au ,Ag Hg 9, 33Oscillatoria perornata var attenate and Scenedesmus quadricauda var longispuia are able to accumulate Fe Zn , Cu and also affected by high concentrations of heavy metalsIn Table 2the toxicity and uptake of heavy metals on Algae are given 6Blue green algae 35Westiellopsis prolifica has significantlyreduced high levels ofNa , K , Ca , Cl , SO42- , PO 43-from Paper mill waste waters . Thiobacillus species are involved in oxidation of sulphide mineralsAspergillus niger and Trichoderma harzianum oxidises sulphides of Cu , Pb , Zn , Co , Mg . Bacillus sp and Vibrio sp reduce Ferromanganese nodules7 Escheria coli can be used to accumulate Ni10Bacterium such as Enterobacter cloacaewill reduce chromate to trivalent chromium which can then be removed using other bioadsorption technology. 8
Zooglea ramigera , common in natural water have been used to accumulate Ni , Fe , Crfrom waste waters 19 Micro organisms involved in bio reduction and bio oxidation of minerals 7 are given in Table 3 & 4 Citrobactor spimmobilised in poly acrylamide gelscavenged large quantities of Cd 16
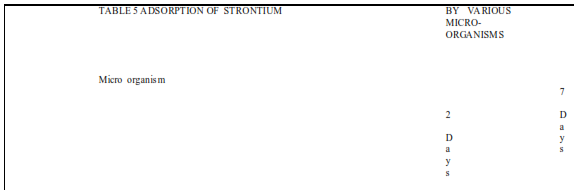
Most research in this area has been associated with heavy metals such as of Cd Cu , Pb , Cr,but a broad range ofmicro organisms has been shown to effectivelyremove metals like Srfrom a typical waste water containing radioactive pollutantsAs seen in Table 5 the distribution coefficients for Sr adsorbtion (quantity of Sr adsorbed per unit mass of dry biosorbent divided by equilibrium concentration of the metalwithin the bulk solution ) 14
IMMOBILIZED MICRO ORGANISMS
The immobilization of micro organisms can bedefined as any technique which limits the free migration of cells There are twotypes of immobilization
(i ) attachement , micro organisms and other cellular materials have a naturalinclination to adhere to surfaces and in this way become immobilised (ii) entrapment where micro organisms are caught in the interstices of fiberous \porous material or physically restrained within solid or porous matrix such as a stabilized gel or a membrane To concentrate large quantities of bio mass , the microbial biomass is entraped into gel matrix like carragenan , alginate , modified bone gel in the form of beads .The gel is generally formed into useful biocatalyst beads by adding the cells as a sus pension to the aqueous solution ofthe gelling material . This material is formed into droplets either by forcingit dropwise through a nozzle or orifice or by dispersing into a non-interacting liquid medium . Immobilization or entrapment of micro organisms is donefor various applications like ethanol fermentation , production of antibiotics and therapeutics . Micro organism Cephalosporium acremonium was immobilized inpoly acrylamide gel , used for theproduction of Cephalosporin ß lactam antibiotic . 27 Citrobactor spimmobilised in poly acrylamide gelscavenged large quantities of Cd16 L.K Jang et al dispensed greenalgae Microcystis in sodium alginate to form a biopolymer gel for therecoveryof Cu2+ andCo 2+ions . 25 Researchers 14, 15 29have discussed theuse ofvarioustypes of bioadsorbentmaterials plant tissue and microbial mass for immobilization in gel matrix for removal of dissolved metals A unique columnar system has been proposed for the use of crosslinked bone gel beads Significantquantities of heavy metal ions will adsorb onbone gel beads in a Fluidized - bed contactor. The loaded beads can easily be removed and regenerated 36
MECHANISM OFBIO ACCUMULATION
Mechanisms by which micro organisms remove metals from solution are (i) extracellular accumulation \ precipitation (ii) cell – surface sorption \complexation (iii) Intracellular accumulation ; process (ii) can occur whether the micro organismis living or dead(i) may be facilitated by micro bial viabilty (iii) process requires micro bial activity
Both active and passive mechanisms are employed by living cells to accumulate dissolved metalsSince , high concentration of heavy metals tend to adversely affect phsiologicalprocesses living cells that adsorb such materials must use [Table 2] mechanisms that ensure that only a limited amount of the metal actually reaches in the interior of the cell where it is most toxic . Many cellular mechanisms result indepositing the metals in cell wall or membrane Citrobactor sp in presence of a suitable phosphate donor is able to immobilise large quantities of Cd , Cu ,Pb ,U byforming insoluble metal phosphates on the cell surface 16 Sulphate reducing bacteria may produce H 2S gas which will react with metal ions to form an insoluble precipitate . 14 Phytochelatins , a class of peptides found in plants are apparently effective inmetal bindingThese peptides are in the range of 5-17 amino acids in length with a carboxyl terminal glycine thatapparently binds metals by thiolate coordination . The Phytochelatins are generated in the living plant tissue that is exposed to metal ions thus providing a means of enhancing the metal adsorption capacityof the biomass
Living cells are affected by metal toxicty and they require the addition of necessary nutrients , maintenance of specific operating conditions . On the other hand nonliving or inactive microbial biomass does not have these requirements and in many cases can be a very effective bioadsorbent for dissolved metals . The cell walls and internal materials within biomass offer abundant sites for metal complexation or micro precipitation . Biological components have amounts of poly saccharides , protiens and lipids that are replete with metal binding functional groups including carboxylate , hydroxy , sulfate , phosphate and amino groups14
It has been shownby several researchers that for some types of microbial biomass ,most of the metal adsorption occurs in the cell ormembrane wall ,this included microbial biomass from fungi such as Ganoderma lucidum which adsorbs Cu 17 , bacteria such as Micrococcus luteus for adsorption of Sr 18 and yeast Saccharomyces cerevisae for U , Zn , Mg , Co .
Strandberg et al studied Saccharomyces cerevisae for U 20 . suggested the role of phosphomannans carboxylic groups of cell wall protein of yeasts in metal binding Yeast cells 23 have been shown to accumulate heavy metals such as Cu , Ni via two distinct process .There is an initial rapid accumulation step that is metabolism and temperature independent ,involves cation binding to negetively charged sites on the cell wall .The outer mannan protein layer of the yeast cell wall as well as the inner glucan -chitin layer are important for heavy metal accumulation Second process is metabolism dependent much slower can accumulate large quantieties of cations .
Researchers have shown that nine species of genus Penicillum had no significant difference in their ability to accumulate metal ions , this accumulation property is associated with a microbial species rather than genus . 21
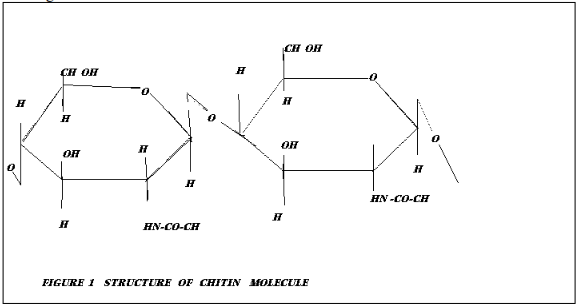
Chitin , an amino polysaccaride with a molecular structure shown in Figure 1 has been postulated as the cell wall component responsible for metal coordination
Tsezos and Volesky have reported results of asystematic study carried out to delineate the mechanism of biosorption of uranium by Rhizopus arrhizus 22.Based on electron microscopy ,X-ray energy dispersion analysis ,and infrared spectrascopic studies , they proposed three processes responsible for metal scavenging :
(i) The first process is complex formation between dissolved uranium ionic species and chitin present in the cell wall of Rhizopus arrhizus .Uranium coordinates to the amino nitrogen of the chitin crystallates and is retained within the cell wall of the mycellium .
(ii) The second process is adsorption of additinal uranium by chitin network close to that complexed by chitin nitrogen .
(ii) The third process is hydrolysis of uranium chitin complex formed by the first process and the precipitation of the hydrolysis product on the cell wall upon which thechitin nitrogen relieved wil re engage further uranium untill accumulation of the hydrolysis product inhibitsthe cycle .
Free radicals are highly unstable species which are traped in the very stable cell wall matrix , this free radical has been attributed to chitin nitogen
Muraleedharan et al 17 have concluded that the free radical present consistently in the biosorbents is not taking part in the metal uptake . However the cell wall matrix which has encampassed and trapped this free radical opens up upon metal uptake indicating its role in metal uptake . The exposed cell wall matrix thus freely interacts with the metal resulting in high removal rates , the free radical can be used to probe for further identification of the stable cell wall components responsible for metal sorption .
Tsezos observed that at pH 4 , uranly uptake by Rhizopus arrhizus was inhibited by competing cations ,while pH 2 no effect was evident J.M..Tobin et al24 studied the effects of cation competition on the uptake of a range of different metals on the fungus Rhizopus arrhizus , They have reported ,the displacement of Ag + ions by UO 2 +2, indicates that the sites of uptake of these metals are the same . In studies where Ag + was in excess the results support the presence of multiplicity of binding sites in the biomass .In the UO 2 +2 - Ag + system it appears that at less than saturation concentrations UO 2 +2 ions bind preferentially to certain sites, leaving others partially unoccupied and available for Ag + binding even at low [Ag + ] \ [UO 2 +2 ] ratios . As this ratio increases ,Ag + ions may increasingly displace theUO 2 +2 ionsfrom the latter type sites .Conversely , at higher UO 2 +2 concentrations UO 2 +2 ions occupy all the sites and Ag+ is not absorbed
Figure 2A Diagrammatic model of the multiciplicity of uptake sites on Rhizopus arrhizus .[ Suggested byJ .M . Tobin and coworkers ]
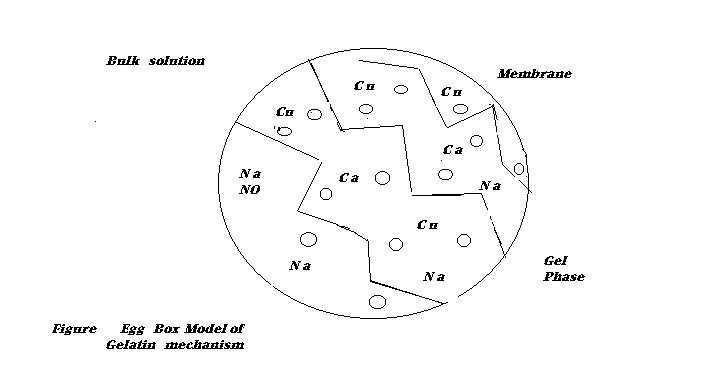
L.K Jang and coworkers dispensed greenalgae Microcystis in sodium alginate to form a biopolymer gel for therecoveryof Cu2+ andCo 2+ions .25 Thealgin contained 4.356 mmol\g uronate residues or repeating monomer unitsAccording to the egg box model of gelatin mechanism , each cavity formed in the alginate gel to trap adivalent metal ion has to involve two carboxylgroups from participating uronate residues . Bone gelatin based gel beads are able to accumulate large quantities of Cu and Sr and thus enhance capacity of immobilized biosorbent 31
CONCLUSION
The preceeding discussion shows that many microbial species exhibit metal binding properties The major potential use for immobilized cells is in biomedical area for production of antibiotics andtherapeutics Waste water treatment is one of the significant application for immobilized cells To evaluate the suitability of a biosorbent for field application in the treatment of metal rich waste waters , it is neccessary to determine the maximum sorption capacity , kinetics of sorption , recovery of the metal and physical state of the biosorbent .
However , not all of them may be suitable , it is better to select the microbial mass which can fulfil the following criteria : (i) the bio sorbent should be produced at low cost and be reusable (ii) uptake and release of the metal should be efficient and rapid (iii) regeneration of the biosorbent should be possible An understanding of the mechanisms by which organisms accumulate metals is important to the developement of microbial processes for the concentration , removal and recovery of the metal from aqueous solutions .A knowledge of the chemical and physiological reactions during metal uptake might enable the specification and control of process parameters to increase the rate of metal accumulation




Discussion of geochemical data
To provide an anaerobic state to support the development of anaerobic microorganisms, all oxygen and other electron acceptors (such as nitrate and sulfate) have tobe consumed.This condition can be achieved by providing substrates such as lactic acid to the saturated zone. HRC is a source of lactic acid. Anaerobic microorganisms metabolize lactic acid to carbon dioxide and water depleting electron acceptors. Subsequently, the oxidation reduction potential (ORP) shifts from a positive to a negative value. Fermentative microorganisms and the HRC-derived lactic acid, in conjunction, form pyruvic acid and acetic acid. This decomposition process provides the hydrogen required for reductive dechlorination (replacing the chlorine in the COCs with hydrogen).
Naturally occurring microorganisms capable of reductive dechlorination then use the hydrogen to progressively remove chlorine atoms from chlorinated hydrocarbon contaminants (i.e., in a simplistic sense, to convert PCE to TCE to DCE to VC to ethane or ethane) (Wiedemeier et al., 1996). Pertinent groundwater geochemical data collected from MW-3 and10 since May 1, 2000 (immediately prior to the HRC injection event). Analysis of perchedgroundwater samples in and around the source area indicate that the saturatedzone is now in an anaerobic condition, which has been promoted by the injection of HRC. ORPmeasurements range from –307 millivolts (mV) in source well MW-3 to -39 mV in MW-10, and ORP has reduced significantly in MW-3 and MW-10 since the application of HRC. An ORP of less than 50 mV is required for effective reductive dechlorination, with values of less than –100 mV being optimal (Wiedemeier et al., 1996). In general, the ORP will reduce only when the electron acceptors (nitrates and sulfates, for example) are consumed. Furthermore, the presence of low quantities of methane in monitoring wells in and around the source area indicates that the aquifer is slightly methanogenic (indicative of anaerobic conditions). Elevated levels of HRC-derived metabolic acids (lactic, propionic, pyruvic, butyric, and acetic acids) in MW-3 and MW-10 indicate that these acids have been effectively released into the shallow aquifer and are being utilized to generate hydrogen within the aquifer to stimulate reductive dechlorination. Permanent gases ethane and ethene have been detected in source well MW-3. The presence of ethane and ethene indicates that the reductive dechlorination pathway has proceeded through DCE and VC to the end-product ethene and/or ethane. Geochemical sampling results indicate conclusively that the HRC injection has been successful in stimulating the reductive dechlorination process.
REFERENCES
Jyotsna Lal , Remediation of toxic metals: biosorption-a viable technology
V.S. Magar and M.E. Kelley (Eds.), In Situ and On-Site Bioremediation—2003. Proceedings of the Seventh
International In Situ and On-Site Bioremediation Symposium (Orlando, FL; June 2003). ISBN 1-57477-139-6, published by Battelle Press, Columbus, OH,
1 Shumate .S.E ; Strandberg GW [1985 ] “Comprehensive Biotechnology” ed Robinson CW ;Howell J A Pergmon Press
2. J .A .Brierly ; G .M. Goyak [1986]Fundamentals and Applied io hydrometallurgy eds R W Lawrence, R M R Bronien ,H G Ebner ElsevierNew York
3. Amutha . R ; Gunasekaran .P [2000] Improved Ethanol production by mixed culture of Saccaromyces diasticus and Zymomonas mobilis from liquified casava starch . Ind . J . Microbiology , 40 , 103- 107
4 . Sawhney .A.L et .al [2000] Degradation of ground water quality due to heavy metals in industrial areas of India -A review , Ind . J. Environ . Protection 20 : 174
5. BIS [1996] Indian Standard : Drinking Water Specification [IS 10500:1991] Bureau of Indian Standard ,New Delhi ,8
6 . Sharad Mittal .; R.M.S .Sengar ; B.D .Kaushik [1992] Uptake and Toxicity of Heavy metals to Algae Ind . J. Microbiology 32, 51-55
7. Pawan Sharma ; Ajit Verma [1991] Microbial reclaimation of metals from ores and industrial waste waters . Ind . J. Microbiology 31 1-26
8 . K. Komori ; A .Rivas ; K . Toda ; H . Ohtake [1990 ] Biological removal of toxic Cr using Enterobacter cloaco strain Biotech & Bioengg , 35, 951 – 54
9.Aksu .Z ; Kutsal .T [1990 ] A comparitive study for biosorption characteristics of heavy metal ions with Chlorella. vulgarisEnviron Tech nol 11, 979-987
10. Ranul Krihna swamy ; David B Wilson[2000] Construction and characterizationof an Escheria coligenetically eng inered for Niaccumulation Appl .Environ Microb 66 , 5383 - 5386
11. Corder S.L ; Reeves .M [1994] Biosorption of Ni in complex aqueous streams by Cyano bacteria Appl. Biochem.Biotechnol 45\46 , 847 - 859
12 . Veglio . F ; Beol/ F; Gasbarro. A [1998] Biosorption of toxic heavymetal ......using freeArthrobacter spProcess Biochem 32, 99-105
13. L .E .Macaskie and A.C.R .Dean [1989 ] Advances in Biotechnological processes Vol 12 eds A . Mizrani , AR Liss New York
14 . Charles .D .Scott and James N. Petersen [1992] Immobilized biosobents for dissolved metals Separation and Purification Technology eds Norman Li , Joseph M Calo , Marcel Dekker Inc New York. Basel .Hong Kong
15. Charles .D .Scott [1987] Immobilised cells A review of recent literature Enzyme .Micro . Techno . , 9 , 66-73
16 .Macaskie , M. ; Dean .A.C.R. , [1986 ] Cd accumulation by immobilised cellsof
Citrobacter sp Biotech & Bioengg , 28 ,1358 –65
17 . Muraleedharan ,T.R ; C. Venkobacchar [1990] Mechanism of biosorption of Cu IIby
Ganoderm luciderm . Biotech & Bioengg [1989 ], 35, 320 – 325
18 . B .D. Faison ; C. A . Cancel ; S.N. Lewis ; H.I. Adler [1990] Appl . Environ Microbiol 56 ,36-49
19 .Norberg . A B ; Pearson . H ; [1984 ] Accumulation of heavy metals by Zooglea ramigeraBiotech & Bioengg 26 , 239
20. Strand berg G.W. ; Shumate S. E ; J. R . Parrot [1981] Microbial cells as Biosorbents for Heavy metals : Accumulation by Saccharomyces cerevisiae ; Pseudomonas aeruginosa Appl . Environ Microbiol, 41 ,237 .
21 . Niu H ; Xu . X .S ;Wang J .H [1993 ] Removal of Lead by Penicillium biomass Biotech & Bioengg 42 ,785-787
22 .Tsezos ; Volesky [1981] Mechanism of biosorption of Uranium by Rhizopus arrhizus Biotech & Bioengg , 23 , 583
23. G. Donmez ; Z .Aksu [2001] Bio accumulation of Cu and Ni by the non adapted and adapted growing Candida sp Wat Res ,35 , 1425 – 1434
24 . Tobin . J.M ; Cooper D .G ; Neufeld .R .J . [1988] The effects of cation competition on metal adsorption by Rhizopus arrhizusBiotech & Bioengg 31, 282 – 286
25 .Jang L K ; Lopez S L; Eastman ;Pryfogie .P [1991] Recovery ofcopper and cobalt by Biopolymer Gels Bio technol & Bioengg ,37 ,266-73
26 .Wulf .C [1984 ] Biotechnology : A Textbook of Industrial Microbiology [ English trans
27 .Saurabh Gupta ; Vinod Bihari ; S. C. Agarwal et. al [2000] studies on free and immobilised cells of Cephalosporium acremonium gel for the production of Cephalosporin [ ß lactam antibiotic ] Ind J . Microbiology40 , 141 -144
28. Macaskie , M ; J. M . Wates ; Dean .A.C.R. , [1987 ] , Cadmium Accumulation by Citrobacterimmobilised on gel and solid supports Biotech & Bioengg30 , 66
29. Good man G.T ; Roberts T . M ; [1971 ]Plants and Soils as indicators of metalsin air .Nature 231 , 287
30.Watson J.S ;Scott C.D ; Fasison B D ; [1990] Emerging Technologies in Hazardous Waste Management ACS Symposium Series no 422 [DW Tedder ; FG Pohland] American Chemical Society Washington
31 . Petersen ,J.N ;Scott C.D ; et al [1990]Accumulation of Cu on to Modified bone gelatin beadsBio technol .Techniqes ,4 ,435 –440
32. Yi-Tin Wang ; Evans ,M. Chirwa [2000] Cr (VI) reduction in continuous flow coculture Bio reactor . J . Environ . Engg . 126 , 300-306
33 . Frencessa Podda ; Paola Zudda et al [2000] Heavy metal co precipitation with Hydro zincite from mine waters caused by photosynthetic micro organisms App . Environ . Micro biology ,11, 5092 -5098
34 . M . Vasanthy ; P . Lakshmana perumala samy ; [2000]Copper accumulation by Pseudomonas sp ; IJEP 20 , 280-283
35 . A .K .Dash ; P .C. Misra [1999] Role of Blue green algaeWestiellopsis prolifica in reducing pollution load from paper mill waste water IJEP ,19 ,1-5
36 J.N. Petersen ; B.H . Davidson ;C.D. Scott ; SL Blankinship [19991] Size changes associated with metal adsorbtion onto Modified bone gelatin beads Biotech & Bioengg38 , 923-928












