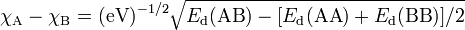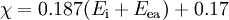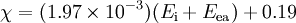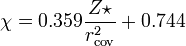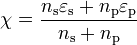1350.8
Atomic sizes across and down the periodic table.
The distance between the nucleus and the outermost electrons of an atom is the atomic
radius that in covalently bonded atom is the covalent radius. The distance between the
nucleus and the outermost electrons of an ion is the ionic radius. Note; Ionic radius
is the radius that an ion has in an ionic crystal, where the ions are packed together to
a point where their outermost electronic orbitals are in contact with each other.
Note: Distances on the atomic scale have traditionally been expressed in Ångstrom
units (1Å = 10–8cm), but nowadays the picometer is preferred; 1 pm = 10–12 m = 10–
10 cm = 100 Å. The radii of atoms and ions are typically in the range 70-400 pm.
Exercise
a) Use the values in Table 2.1 to plot an excel graph for the atomic number (hori-
zontal/X-axis) versus the Van der waals/atomic radius (pm) (vertical/Y-axis) for
any three (3) periods shown in the table.
b) Use the values in Table 2.1 to plot an excel graph for the atomic number (hori-
zontal/X-axis) versus the Van der waals/atomic radius (pm) (vertical/Y-axis) for
the groups shown in the table.

African Virtual University
Solution
The two plots in the exercise 2.1 above should show that the Elements in the same
period show trends in atomic radius, from top to bottom in a group, the atomic radii
of the elements increase. Since there are more filled energy levels, electrons are found
farther from the nucleus. Moving left to right across a period, atomic radius usually
decreases. This occurs because each successive element has an added proton and
electron which causes the electron to be drawn closer to the nucleus.
Melting and boiling points across and down the periodic table
The melting point of an element or compound means the temperatures at which
the solid form of the element or compound is at equilibrium with the liquid
form. While the boiling point of an element or compound means the tempera-
ture at which the liquid form of an element or compound is at equilibrium with
the gaseous form. We usually presume the air pressure to be 1 atmosphere.
Exercise
c) Use the values in Table 2.1 to plot an excel graph for the atomic number (hori-
zontal/X-axis) versus the melting points (vertical/Y-axis) for any three (3) periods
shown in the table.
d) Use the values in Table 2.1 to plot an excel graph for the atomic number (hori-
zontal/X-axis) versus the melting points (vertical/Y-axis) for the groups shown
in the table.
For example consider Period 3. The trends in melting points and boiling points going
across Period 3 are not straightforward, and need more detailed consideration than
the trends in Group 2: We note that, the melting points generally increase going from
sodium to silicon, then decrease going to argon (with a “bump” at sulphur). Boiling
points generally increase going from sodium to aluminium, then decrease to argon
(again with a “bump” at sulphur).
Explanation
Sodium, magnesium and aluminium are all metals. They have metallic bonding, in
which positive metal ions are attracted to delocalised electrons. Going from sodium
to aluminium: a) the charge on the metal ions increases from +1 to +3 (with magne-
sium at +2), b) the number of delocalised electrons increases, c) so the strength of the
metallic bonding increases, and d) the melting points and boiling points increase.
Silicon is a metalloid (an element with some of the properties of metals and some of
the properties of non-metals). Silicon has a very high melting point and boiling point
because: a) all the silicon atoms are held together by strong covalent bonds, and b)
which need a very large amount of energy to be broken.

African Virtual University
Phosphorus, sulphur, chlorine and argon, these are all non-metals, and they exist
as small, separate molecules. Phosphorus, sulphur and chlorine exist as simple mo-
lecules, with strong covalent bonds between their atoms. Argon exists as separate
atoms (it is monatomic). Their melting and boiling points are very low because: a)
when these four substances melt or boil, it is the van der Waals’ forces between the
molecules which are broken, b) are very weak bonds, and c) so little energy is needed
to overcome them.
Sulphur has a higher melting point and boiling point than the other three because
sulphur does exist as S molecules:
8
Electronegativity (Xp) across and down the periodic table
Electronegativity, symbol χ, first proposed by Linus Pauling in 1932, is a chemical
property that describes the power of an atom (or, more rarely, a functional group) to
attract electrons towards itself. Electronegativity, as it is usually calculated, is not
strictly an atomic property, but rather a property of an atom in a molecule. Electrone-
gativity of an element varies with its chemical environment, though it is considered
to be a transferable property, meaning that similar values will be valid in a variety
of situations.
Electronegativity cannot be directly measured and must be calculated from other
atomic or molecular properties. Several methods of calculation have been proposed
and, although there may be small differences in the numerical values of the electro-
negativity, all methods show the same periodic trends between elements.
Exercise
a) Use the values in Table 2.1 to plot an excel graph for the atomic number (hori-
zontal/X-axis) versus corresponding Pauli electronegativity (Xp) (vertical/Y-axis)
for any three (3) periods shown in the table.
b) Use the values in Table 2.1 to plot an excel graph for the atomic number (horizon-
tal/X-axis) versus the coresponding Pauli electronegativity (Xp) (vertical/Y-axis)
for the groups shown in the table.
Solution
For all the periods across the table, electronegativity increases from left to right.
However, it decreases down any given group (See also Figure 2.1). You have to
ignore inert gases group. It doesn’t have an electronegativity, because it is not good
at forming bonds.

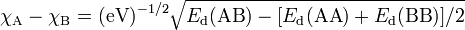
African Virtual University
Methods of calculation
Pauling electronegativity
Pauling first proposed the concept of electronegativity in 1932 as an explanation of
the fact that the covalent bond between two different atoms (A–B) is stronger than
would be expected by taking the average of the strengths of the A–A and B–B bonds.
Consider a bond between two atoms, A and B. Each atom may be forming other bonds
as well. If the atoms are equally electronegative, both have the same tendency to attract
the bonding pair of electrons, and so it will be found on average half way between
the two atoms. To get a bond like this, A and B would usually have to be the same
atom. You will find this sort of bond in, for example, H or Cl molecules.
2
2
According to valence bond theory, of which Pauling was a notable proponent, the
«additional stabilization» of the heteronuclear bond is due to the contribution of ionic
canonical forms to the bonding.
The difference in electronegativity between atoms A and B is given by:
where the dissociation energies, E , of the A–B, A–A and B–B bonds are expressed
d
in electronvolts, the factor (eV)−½ being included to ensure a dimensionless result.
Hence, the difference in Pauling electronegativity between hydrogen and bromine is
0.73 (dissociation energies: H–Br, 3.79 eV; H–H, 4.52 eV; Br–Br 2.00 eV).
As only differences in electronegativity are defined, it is necessary to choose an
arbitrary reference point in order to construct a scale. Hydrogen was chosen as the
reference, as it forms covalent bonds with a large variety of elements: its electronega-
tivity is fixed at 2.20 on a relative scale running from 0.7 to 4.0. It is also necessary to
decide which of the two elements is the more electronegative (equivalent to choosing
one of the two possible signs for the square root). This is done by «chemical intuition»:
in the above example, hydrogen bromide dissolves in water to form H+ and Br− ions,
so it may be assumed that bromine is more electronegative than hydrogen.
To calculate Pauling electronegativity for an element, it is necessary to have data on
the dissociation energies of at least two types of covalent bond formed by that ele-
ment. Allred updated Pauling’s original values in 1961 to take account of the greater
availability of thermodynamic data, and it is these «revised Pauling» values of the
electronegativity which are most usually used.

African Virtual University
figure 2.1.Periodic trends of electronegativity using the Pauling scale
The variation of Pauling electronegativity ( y-axis) as one descends the main groups
of the Periodic table from the second period to the sixth period.
mulliken electronegativity
Mulliken proposed that the arithmetic mean of the first ionization energy and the elec-
tron affinity should be a measure of the tendency of an atom to attract electrons. As
this definition is not dependent on an arbitrary relative scale, it has also been termed
absolute electronegativity, with the units of kilojoules per mole or electronvolts.
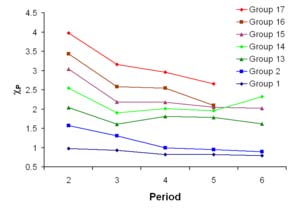
The correlation between Mulliken electronegativities ( x-axis, in kJ/mol) and Pauling
electronegativities ( y-axis).

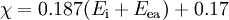
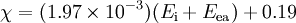
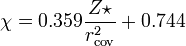
African Virtual University
However, it is more usual to make use of a linear transformation to transform these
absolute values into values which resemble the more familiar Pauling values. For
ionization energies and electron affinities in electronvolts,
and for energies in kilojoules per mole,
The Mulliken electronegativity can only be calculated for an element for which the
electron affinity is known, fifty-seven elements as of 2006.
Allred-Rochow electronegativity
Allred and Rochow considered that electronegativity should be related to the charge
experienced by an electron on the «surface» of an atom: the higher the charge per
unit area of atomic surface, the greater the tendency of that atom to attract electrons.
The effective nuclear charge, Z* experienced by valence electrons can be estimated
using Slater’s rules, while the surface area of an atom in a molecule can be taken to
be proportional to the square of the covalent radius, r . When r is expressed in
cov
cov
ångströms,
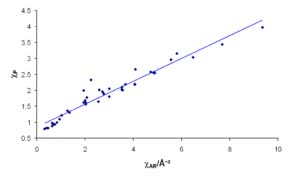
The correlation between Allred-Rochow electronegativities ( x-axis, in Å−2) and
Pauling electronegativities ( y-axis).

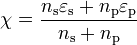
African Virtual University
The other methods are;
1. Sanderson electronegativity: Sanderson has also noted the relationship between
electronegativity and atomic size, and has proposed a method of calculation
based on the reciprocal of the atomic volume. With a knowledge of bond lengths,
Sanderson electronegativities allow the estimation of bond energies in a wide
range of compounds. Also Sanderson electronegativities are used for different
investigations in organic chemistry.
2. Allen electronegativity: Perhaps the simplest definition of electronegativity is
that of Allen, who has proposed that it is related to the average energy of the
valence electrons in a free atom.
where ε are the one-electron energies of s- and p-electrons in the free atom and
s,p
n are the number of s- and p-electrons in the valence shell. It is usual to apply a
s,p
scaling factor, 1.75×10−3 for energies expressed in kilojoules per mole or 0.169 for
energies measured in electronvolts, to give values which are numerically similar to
Pauling electronegativities.
In general, electronegativity increases on passing from left to right along a period,
and decreases on descending a group. Hence, fluorine is undoubtedly the most elec-
tronegative of the elements while caesium is the least electronegative, at least of those
elements for which substantial data are available.
There are some exceptions to this general rule. Gallium and germanium have higher
electronegativities than aluminium and silicon respectively because of the d-block
contraction. Elements of the fourth period immediately after the first row of the
transition metals have unusually small atomic radii because the 3d-electrons are not
effective at shielding the increased nuclear charge, and smaller atomic size correlates
with higher electronegativity.

African Virtual University
Correlation of electronegativity with oxidation number
In inorganic chemistry it is common to consider a single value of the electronegativity
to be valid for most «normal» situations. While this approach has the advantage of
simplicity, it is clear that the electronegativity of an element is not an invariable atomic property and, in particular, increases with the oxidation state of the element.
Consider:
1. What happens if two atoms of equal electronegativity bond together?
Consider a bond between two atoms, A and B. Each atom may be forming other bonds
as well as the one shown - but these are irrelevant to the argument. If the atoms are
equally electronegative, both have the same tendency to attract the bonding pair of
electrons, and so it will be found on average half way between the two atoms.
Reminder: To get a bond like this, A and B would usually have to be the same atom.
You will find this sort of bond in, for example, H or Cl molecules.
2
2
This sort of bond could be thought of as being a «pure» covalent bond - where the
electrons are shared evenly between the two atoms. The molecule is said to be non-
polar.
A polar bond is a covalent bond in which there is a separation of charge between one
end and the other - in other words in which one end is slightly positive and the other
slightly negative. Examples include most covalent bonds. The hydrogen-chlorine bond
in HCl or the hydrogen-oxygen bonds in water are typical. In general, the greater the
difference in electronegativity between two atoms, the more polar the bond that will
be formed between them, with the atom having the higher electronegativity being at
the negative end of the dipole
2. What happens if B is slightly more electronegative than A?
B will attract the electron pair rather more than A does.
That means that the B end of the bond has more than its fair share of electron den-
sity and so becomes slightly negative. At the same time, the A end (rather short of
electrons) becomes slightly positive.
3. What happens if B is a lot more electronegative than A?
In this case, the electron pair is dragged right over to B’s end of the bond. To all
intents and purposes, A has lost control of its electron, and B has complete control
over both electrons. Ions have been formed.
The implication of all this is that there is no clear-cut division between covalent and
ionic bonds. In a pure covalent bond, the electrons are held on average exactly half
way between the atoms. In a polar bond, the electrons have been dragged slightly
towards one end.
How far does this dragging have to go before the bond counts as ionic? There is
no real answer to that. You normally think of sodium chloride as being a typically

African Virtual University
ionic solid, but even here the sodium hasn’t completely lost control of its electron.
Because of the properties of sodium chloride, however, we tend to count it as if it
were purely ionic.
Lithium iodide, on the other hand, would be described as being «ionic with some
covalent character». In this case, the pair of electrons hasn’t moved entirely over to
the iodine end of the bond. Lithium iodide, for example, dissolves in organic solvents
like ethanol - not something which ionic substances normally do.
In Summary
- No electronegativity difference between two atoms leads to a pure non-polar
covalent bond.
- A small electronegativity difference leads to a polar covalent bond.
- A large electronegativity difference leads to an ionic bond.
- ΔΧ ≤ 1.2 covalent bond
- ΔΧ ≈ 1.5 moderately ionic
- ΔΧ ≥ 2.0 ionic
Quiz. Is CHCl polar or non-polar?.
3
Answer: CHCl is polar. The hydrogen at the top of the molecule is less electrone-
3
gative than carbon and so is slightly positive. This means that the molecule now has
a slightly positive «top» and a slightly negative «bottom», and so is overall a polar
molecule.
Explaining the patterns in electronegativity
The attraction that a bonding pair of electrons feels for a particular nucleus depends
on: the number of protons in the nucleus, the distance from the nucleus, and the
amount of screening by inner electrons.
Why does electronegativity increase across a period?
Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the
noble gas, argon). Think of sodium chloride as if it were covalently bonded.
Both sodium and chlorine have their bonding electrons in the 3-level. The electron
pair is screened from both nuclei by the 1s, 2s and 2p electrons, but the chlorine
nucleus has 6 more protons in it. It is no wonder the electron pair gets dragged so far
towards the chlorine that ions are formed.
Electronegativity increases across a period because the number of charges on the
nucleus increases. That attracts the bonding pair of electrons more strongly.
Why does electronegativity fall as you go down a group?
Think of hydrogen fluoride and hydrogen chloride.
The bonding pair is shielded from the fluorine’s nucleus only by the 1s2 electrons.
In the chlorine case it is shielded by all the 1s22s22p6 electrons. In each case there

African Virtual University 0
is a net pull from the centre of the fluorine or chlorine of +7. But fluorine has the
bonding pair in the 2-level rather than the 3-level as it is in chlorine. If it is closer to
the nucleus, the attraction is greater.
As you go down a group, electronegativity decreases because the bonding pair of
electrons is increasingly distant from the attraction of the nucleus.
nOTE; Positive ions can have the effect of polarising (electrically distorting) nearby
negative ions. The polarising ability depends on the charge density in the positive
ion.
Polarising ability increases as the positive ion gets smaller and the number of charges
gets larger.
As a negative ion gets bigger, it becomes easier to polarise. For example, in an iodide
ion, I-, the outer electrons are in the 5-level - relatively distant from the nucleus. A
positive ion would be more effective in attracting a pair of electrons from an iodide
ion than the corresponding electrons in, say, a fluoride ion where they are much
closer to the nucleus.
Aluminium iodide is covalent because the electron pair is easily dragged away from
the iodide ion. On the other hand, aluminium fluoride is ionic because the aluminium
ion can’t polarise the small fluoride ion sufficiently to form a covalent bond.
Ionization EnergY/POTENTIAL
The ionization energy potential or E of an atom or molecule is the energy required
I
to remove one mole of electrons from one mole of isolated gaseous atoms or ions.
More generally, the nth ionization energy is the energy required to strip of an n th mole of electrons after the first (n+1) mole of electrons have already been removed. It is
considered as a measure of the «reluctance» of an atom or ion to surrender an electron,
or the «strength» by which the electron is bound; the greater the ionization energy,
the more difficult it is to remove an electron.
Usually, any subsequent ionization energy involves removing an electron from an
orbital closer to the nucleus. Electrons in the closer orbital experience greater forces
of electrostatic attraction, and thus, require more energy.
Generally, any subsequent ionization energy involves removing an electron from an
orbital closer to the nucleus in an already more positively charged ion. Electrons in
the closer orbital experience greater forces of electrostatic attraction, and thus, require
more energy to be removed.
Exercise
Use the values in Table 2.1 to plot an excel graph for the atomic number (horizontal/
X-axis) versus corresponding 1st ionization energies (vertical/Y-axis) for any three
(3) periods shown in the table. Repeat the same for 1st ionization energies for the
groups shown in the table.









African Virtual University
Solution
It is a minimum for the alkali metals which have a single electron outside a closed
shell. It generally increases across a row (period) with the periodic maximum for the
noble gases which have closed shells. For example, sodium requires only 496 kJ/mol
or 5.14 eV/atom to ionize it while neon, the noble gas immediately preceding it in
the periodic table, requires 2081 kJ/mol or 21.56 eV/atom. The ionization energy is
one of the primary energy considerations used in quantifying chemical bonds