(6.6)
K
cr − o
where С – specific heat capacity of concrete kJ/kg⋅K; t – maximal
cr
(critical) temperature (Celsius) of hardened concrete; К – factor
depending on conditions of concrete cooling (K≤1); t – temperature
о
(Celsius) of the fresh concrete after its finishing; ρ – concrete density,
kg/m3.
116





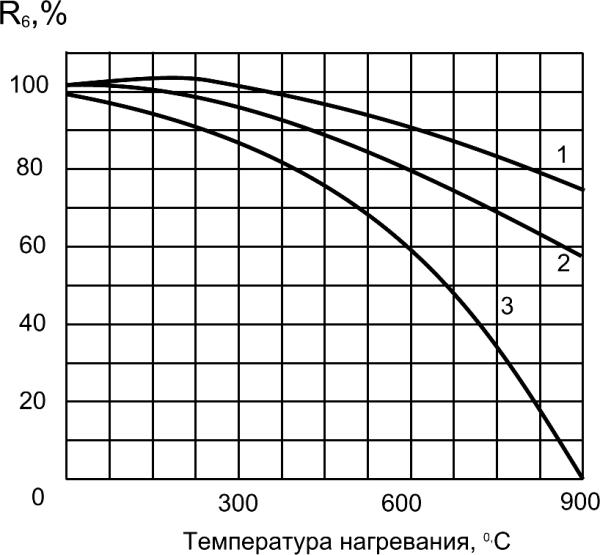
Strength, %
Intensive destructive processes
begin at heating concrete to
temperature more than 200°C.
For heat resistance increase,
finely divided mineral
admixtures can be added into
cement or concrete mixes, that
chemically react with calcium
oxide, resist to heats and
reduce shrinkage of cement
Temperature of heating, 0C
stone at heating.
Fig.6.5. Effect of temperature on strength of
concrete:
1 – Portland cement 70% + Trepel 30%;
2 – Portland cement 70% + Pumice 30%;
3 – Portland cement
117





6.3. Permeability
Permeability of concrete characterizes its ability to conduct gases and liquids at
a certain pressure difference. Permeability of concrete is defined by a factor of
permeability - the quantity of a liquid getting through unit of the area of the
specimen in unit of time at a gradient of a pressure equal 1.
In concrete there are capillaries of the various size, therefore various
mechanisms of moving of gas and liquids can simultaneously operate.
Watertightness
Two normative characteristics of watertightness are possible to use:
1. Maximal pressure of water (W, MPa) which standard specimens with height
and diameter 150 mm can sustain without water infiltration.
2. Coefficient of water filtration through a concrete defines the quantity of water
getting through unit of the area for a time unit, at a gradient of water pressure
equal 1.
The coefficient of water filtration through concrete can be used for
determination of permeability for other liquids:
(К /К) = (η/η ), (6.7)
f
w
where К and η - coefficient of permeability and viscosity of liquid different from
water; К and η - coefficient of filtration and water viscosity.
f
w
118





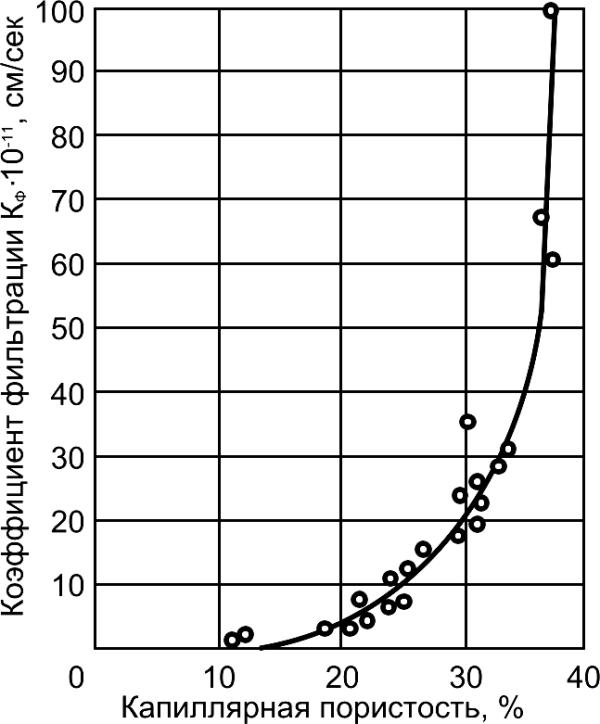
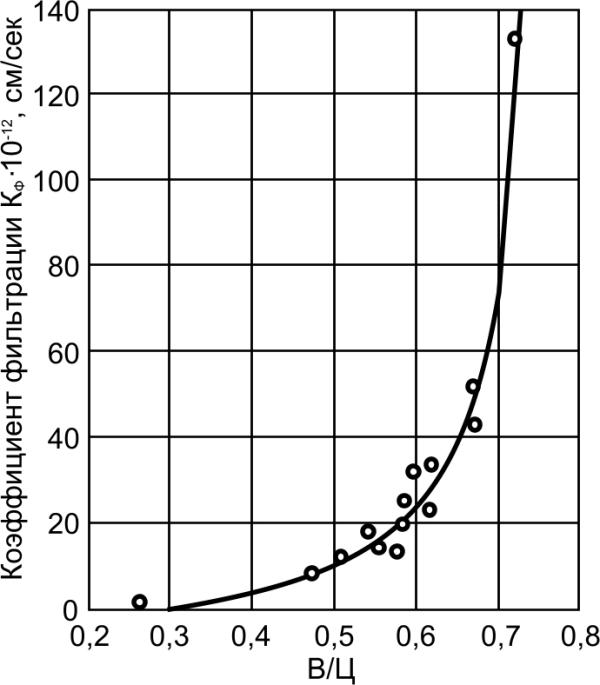
/sec
/sec
, cm1
, cm
-1
-12
⋅10
⋅10
K f
K f
on
on
ltrati fi
filtrati
t of
t ofenci
oefficienC
oeffiC
Capillary porosity, %
Water-cement ratio
Fig.6.6. Relationship between
Fig.6.7. Relationship between
permeability and capillary porosity of
permeability and water-cement ratio of
the cement stone
the cement stone
119





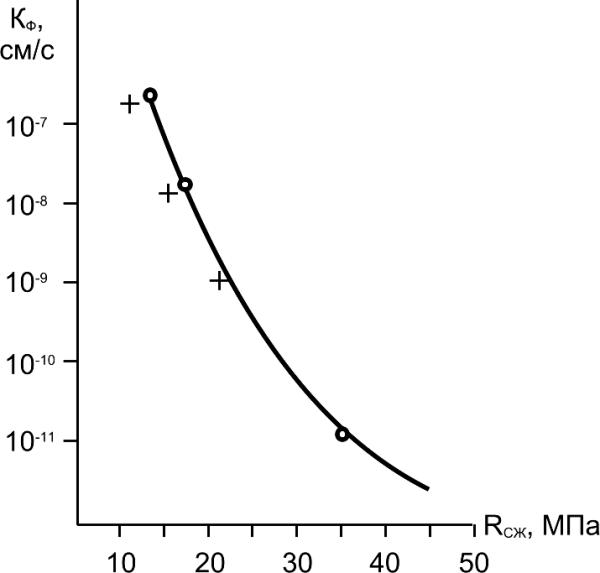
As it is experimentally shown, relationship between coefficient of concrete
filtration (K ) and its compressive strength (R
) is defined as:
f
cmp
К
К
R m
, (6.8)
f =
w
cmp
Kf,
cm/sec
where К and m - factors which values
w
are determined by features of concrete
mixtures, conditions and duration of
hardening, etc.
Effective way of decreasing of concrete
permeability is adding organic or
inorganic admixtures into concrete mix.
R
As organic materials apply surfacecmp, MPa
active and polymeric admixtures .
Fig. 6.8. Relationship between coefficient
Inorganic materials for decrease of
of filtration of concrete (Kf) and
permeability are presented by various
compressive strength (Rcmp):
"+" – From Elbakidze,
salts, clays and active mineral
"ο"–Our experimental data
admixtures (pozzolans).
)
120





After producing concrete's constructions, decrease in its permeability can be
reached by processing of concrete surface by waterproof substances and the
substances chemically reacting with minerals of cement stone with formation of
insoluble compounds or covering surface by protective materials.
6.4. Corrosion resistance
Degree of aggressive effect of an environment is defined by its chemical
composition and a complex of the factors describing conditions of contact of
environment and concrete.
Cement stone consists of alkaline chemical compounds, therefore the most
intensive corrosion of concrete occurs at influence of the environment
containing water solutions of acids on it. Salts, inorganic and organic
substances can be also aggressive to concrete.
The degree of aggressive influence of liquids depends on concentration of
hydrogen ions (pH), amount of carbonic acid (CO ), salts, caustic alkalis,
2
sulfates. Oils and solvents also are aggressive liquids.
121





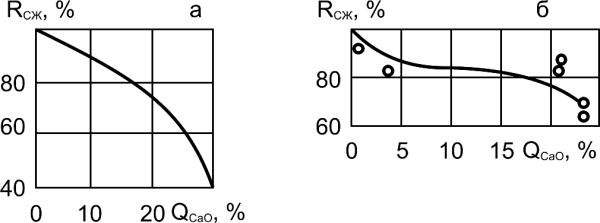
Rcmp, %
A
Rcmp, %
B
QCaO, %
QCaO, %
Рис. 6.10. Effect of dissolution of calcium hydroxide on
compressive strength of cement stone (A) and concrete (B):
QCaO - Amount of dissolved calcium hydroxide, %;
Rcmp – Compressive strength of cement stone and concrete, %
From Moskvin classification, dissolution processes of lime and its washing
away from concrete concern to corrosion of first type.
122





Corrosion of the second type is caused by chemical reactions between the
products of hydration of cement and acids or salts which affect concrete.
Calcium salts of usually well water-soluble appear as a result of action of acids.
Corrosion of the second type is also caused by magnesium salts, often
presents in large amount in underground and sea water (15.5...18% from total
salts content). At magnesia corrosion appears amorphous mass of Mg(OH)2
decreasing strength of concrete along with soluble salts.
Corrosion of the third type develops in concrete from internal stress due to
accumulation of insoluble salts in the capillaries of concrete.
The most widespread corrosion of this type is sulfate corrosion which takes
place in cement stone under action of ions.
2−
SO 4
123





Ettringite appears in the cement stone under the action of sulfate water:
СаО
3
⋅ Al O ⋅ 6Н О
2
3
2
+ (
3 СаSO ⋅ 2H O
4
2 ) + 19H О
2
=
СаО
3
⋅ Al O ⋅ Са
3 SO ⋅ H
31
O
2
3
4
2
Volume expansion and concrete destruction are often caused by
ettringite formation.
Active mineral admixtures (pozzolans) essentially increase sulfate
resistance due to chemical reaction with calcium hydroxide.
2−
Water containing more than 1000 mg/Litre ions SO4
cause mainly gypsum corrosion due to accumulation of gypsum in
capillaries of the cement stone.
Destructions of concrete under influence of vegetative and animal
organisms are called biological damages.
124





Durability of concrete in the terms of influence of aggressive environment is
provided by application of concrete with a high density, by use initial components
with the proper chemical composition and application at a necessity the special
measures of concrete's defense (application of isolating materials, admixtures
etc.).
Special kind of the aggressive environment for concrete is ionizing radiation.
Structures of nuclear reactors are exposed to the greatest degree ionizing
radiation. Ability of concrete to keep their properties after radiation action is
called radiating resistance.
125

























































