G0/G1 phase. PDEE increased the apoptotic cell population from 11.4% in case of control to
49.6% at 100 μg/ml. Further, PDEE induces loss of mitochondrial membrane potential (∆Ψm)
to 99.5% at 100 μg/ml from 24.4% in control cells. These primary results depict the free radical
scavenging potential and anticancer activity of P. denticulata extracts. These findings may
serve as foundation to develop an anticancer drug from medically important P. denticulata.
Keywords: Apoptosis; DNA damage; DPPH; MiaPaCa-2 cells; Primula denticulata
1. Introduction
cases and 13.5 million deaths by 2030.
Pancreatic cancer is the fourth most
Cancer after cardiovascular dis-
common cause of cancer-related deaths
eases is the second leading mortality
across the world with incidence equalling
cause and is rapidly becoming a global
mortality and continues to pose an enor-
pandemic. The worldwide incidence and
mous challenge to clinicians and cancer
mortality of cancer in 2008 were 12.66
scientists (Hariharan et al., 2008). Among
and 7.56 million cases respectively. Ac-
all pancreatic cancers, pancreatic ductal
cording to (World Health Organization,
adenocarcinoma (PDAC) is the most
2010) report, the global cancer burden is
common epithelial, exocrine pancreatic
expected to nearly double to 21.4 million
malignancy, representing more than 80%
ISBN: 978-967-14475-3-6; eISBN: 978-967-14475-2-9 512
Biotech Sustainability (2017)
Free Radical Scavenging Potential and Anticancer Activity of Primula sp. Wani et al.
of the malignant neoplasms of the pancre-
tine,
podophyllotoxin,
camptothecin,
as (Alexakis et al., 2004).
combretastatins, flavopiridol, bruceatin
Consumption of fruits and vegeta-
etc, with diverse chemical structures have
bles is known to impart reduction in the
been isolated from plants. Several biolog-
incidence of ischemic heart disease and
ically active analogues such as taxotere,
some types of cancer, particularly stom-
isotaxel (taxol analogues) topotecan, iri-
ach, oesophagus, lung, oral cavity and
notecan, rubitecan, lurtotecan, 9-Amino
pharynx, endometrial, pancreas and colon
CPT (camptothecin analogues), etoposide,
cancers (Mathew et al., 2004). Similarly
teniposide (podophyllotoxin analogues),
natural antioxidant supplements (ascorbic
vinorelbine, hydravin (Vinca alkaloid de-
acid, tocopherols, anthocyanin, β-carotene
rivatives) have been synthesised from the-
and other polyphenols have been associ-
se front line anticancer lead molecules
ated with lower incidences of cancers and
(Vandana et al., 2005). As a result, em-
cancer related diseases (Fleischauer et al.,
phasis has now been shifted towards the
2003). During some pathophysiological
screening of apoptotic inducers from nat-
conditions, excess amount of reactive ox-
ural sources particularly from plants in
ygen species (ROS) is being generated by
the form of extracts or as isolated com-
certain external agents such as UV-
pounds that specifically increase apoptot-
radiations, drugs, pollution, other xenobi-
ic cell death in cancerous cells.
otics and as well as by endogenous chem-
Primula denticulata Sm. (Primu-
icals, especially stress hormones (adrena-
laceae) is an important member of genus
lin and noradrenalin). The superoxide
primula, which represent more than 400
dismutase (SOD) and other defence
species (Richards, 1993). P. denticulata is
mechanisms in living organisms are una-
commonly known as drumstick primula
ble to scavenge excess of ROS complete-
or tooth-leaved primula. P. denticulata is
ly, which causes damage to cellular mole-
20-30 cm tall perennial rarely annual, de-
cules such as DNA, RNA, enzymes, lipids
ciduous, clump-forming plant with com-
etc. that results in fluidity of bio-
pact heads of many flowers. The plant is
membranes (Dean and David, 1993) and
widely distributed from eastern Afghani-
development of degenerative diseases in-
stan and northern Pakistan, across the
cluding caners, cardiovascular, neuro-
Himalaya to Yunnan, Sichuan and Gui-
degenerative, Alzheimer’s and inflamma-
zhou in China. In Kashmir Himalaya, the
tory diseases (Shahidi et al., 1992; Gerber
species is widely distributed (Map-1). The
et al., 2002; Di Matteo and Esposito,
species thrives best in moist, shady
2003; Sreejayan and Rao, 1996). Hence
slopes, mostly near melting glaciers and
there is growing interest in natural poly-
moist meadows, ranging in altitude from
phenolic compounds, present in medicinal
2100 – 4050 meters.
and dietary plants that might help attenu-
ate oxidative damage (Silva et al., 2005).
2. Materials and methods
The increased incidence of differ-
ent types of cancers during the last few
2.1. Chemicals
decades and the modern techniques for
Sulphorhodamine-B
(SRB),
separation, structure elucidation, screen-
RPMI-1640 medium, fetal bovine serum
ing and combitorinial synthesis have led
(FBS), streptomycin, sodium bicarbonate,
to the development of new anticancer
5-Fluorouracil, paclitaxel, gentamycin
drugs, drug combinations and chemother-
sulphate,
trypsin,
1,1-diphenyl-2-
apy strategies by exploration of enormous
picrylhydrazyl (DPPH), folin–Ciocalteu
pool of biological, synthetic and natural
reagent, catechin, gallic acid were pro-
products (Mukherjee et al., 2001). So far
cured from Sigma-Aldrich. Trichloroace-
several potential anticancer lead mole-
tic acid (TCA), butylated hydroxytoluene
cules such as taxol, vincristine, vinblas-
(BHT), thiobarbituric acid (TBA), hydro-
ISBN: 978-967-14475-3-6; eISBN: 978-967-14475-2-9 513

Biotech Sustainability (2017)
Free Radical Scavenging Potential and Anticancer Activity of Primula sp. Wani et al.
N
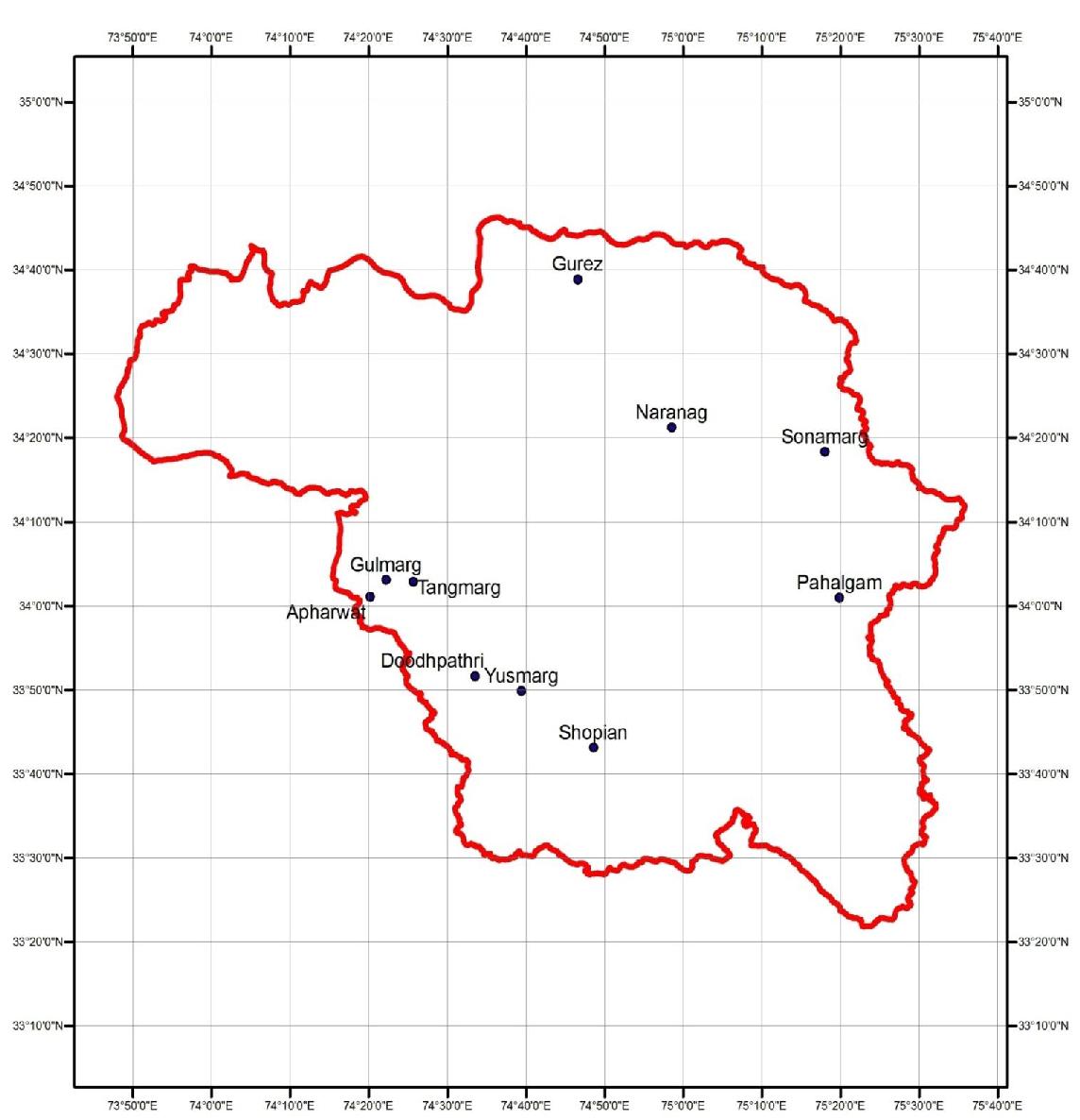
Map 1: Distribution of Primulla denticulata Sm. in Kashmir Himalaya, J&K- India.
gen peroxide (H2O2), ferric chloride, di-
tude of 2650 m. The healthy plant species
methyl sulfoxide (DMSO), potassium fer-
were randomly collected by hand-picking
ricyanide were purchased from Merck.
and later identified by Dr. Anzar A.
The other reagents used were all of ana-
Khuroo at department of Botany, Univer-
lytical grade.
sity of Kashmir. A specimen under
voucher number KASH-1743 was pre-
2.2. Collection and identification of plant
served for future reference.
Primula denticulata Sm. at flow-
ering stage was collected from Gulmarg
2.3. Extract preparation
region of Kashmir Himalaya (latitude
Fresh and healthy leaves of P.
34°3'27" N; longitude 74°23'9" E) at alti-
denticulata (1Kg) were cleaned with dou-
ISBN: 978-967-14475-3-6; eISBN: 978-967-14475-2-9 514
Biotech Sustainability (2017)
Free Radical Scavenging Potential and Anticancer Activity of Primula sp. Wani et al.
ble distilled water, dried under shade (25±
pable of donating hydrogen or electron.
2°C) for 5-6 days. The dried plant materi-
100 μl of different concentrations (100-
al was ground to powder form. The plant
600 μg/ml) of plant extract or standard
powder was extracted in soxhelt apparatus
antioxidant was added to 1 ml DPPH so-
using ethanol as solvent at desirable tem-
lution (0.5 mM). The solution was slight-
perature. The filtered extract was concen-
ly shaken and kept stand for 30 min at
trated using Buchi rotavapour and stored
room temperature under dark conditions.
in glass vials at 4oC until used.
The yellow colour solution was read at
517 nm against ethanol (Brand-Williams
2.4. Estimation of total phenolics
et al., 1995). The free radical inhibition
The total phenolic content in leaf
was calculated as:
extract of P. denticulata was determined
Percentage inhibition = [(Ac-As)/Ac] x
by Folin–Ciocalteu method as adopted by
100
Slinkard and Singleton (1977) with slight
Where, Ac and As are the absorbance of
modifications. To 0.2 ml of plant extract
control and sample respectively
(1mg/ml) was added to 2.5 ml of 10% di-
Butylated hydroxytoluene and α- tocoph-
luted Folin–Ciocalteu reagent and 2 ml of
erol were used as positive control.
2.5% aqueous Na2CO3. The reaction mix-
●
ture was incubated at room temperature
2.7. Hydroxyl radical (HO ) scavenging
with intermittent shaking. The blue colour
assay
solution was read at 765 nm on UV–
Deoxyribose assay was used to
visible spectrophotometer. The absorb-
evaluate the hydroxyl radical scavenging
ance of solution was compared against
potential of P. denticulata leaf extract
●
standard Gallic acid (50 mg %) calibra-
(Halliwell et al., 1987). The HO generat-
tion curve.
ed in Fenton reaction attack deoxyribose
to form products that upon heating with
2.5. Estimation of total flavonoids
thiobarbituric acid at low pH yield a pink
The aluminium chloride colori-
chromogen (TBARS). A reaction mixture
metric method as described by Mcdonald
containing deoxyribose (25 mM), FeCl3
et al., (2001) was used to determine the
(10 mM), ascorbic acid (100 mM), H2O2
total flavonoid content of leaf extract. The
(2.8 mM) in 10 mM KH2PO4 (pH 7.4)
principle of this method is based on fla-
with or without plant extract at various
vonoid–aluminium complex formation,
concentrations (20-120 µg/ml) and incu-
which shows absorbance maximum at 430
bated at 37 0C for 1h. Then 1 ml of TBA
nm. Briefly 0.5 ml (1mg/ml) of extract
(1% w/v) and 1 ml of TCA (3% w/v)
was mixed with 1.5 ml of ethanol, 0.1 ml
were added and heated at 100 0C for 20
of 10% AlCl3, 0.1 ml of 1M potassium
min. Absorbance of TBARS was read at
acetate and 2.8 ml of distilled water. After
532 nm. Deoxyribose oxidation inhibition
5 min of incubation, the absorbance was
was calculated as:
read at 430 nm. Flavonoid concentration
Percentage inhibition = [(A-B)/A] ×100
was expressed as milligrams of catechin
Where, A is malonaldehyde produced
equivalents per gram dry weight.
when treated with extract and B is malo-
naldehyde produced without extract. Bu-
2.6. DPPH assay
tylated hydroxytoluene and α- tocopherol
DPPH assay is one of the most ex-
were taken as the positive control.
tensively used method for determining the
antioxidant potential of any biological
2.8. DNA damage assay
sample. DPPH is a purple stable free radi-
The Prevention of oxidative DNA
cal which is reduced to yellow colour
damage by PDEE was determined by
complex 1,1-diphenyl-2-picrylhydrazine
method as previously described by Ghan-
(DPPH-H) by compounds which are ca-
ta et al., (2007). Calf thymus DNA
ISBN: 978-967-14475-3-6; eISBN: 978-967-14475-2-9 515
Biotech Sustainability (2017)
Free Radical Scavenging Potential and Anticancer Activity of Primula sp. Wani et al.
(0.37μg) with and without plant extract
at room temperature. The optical density
(10, 30, 50, 80 and 100 μg) was incubated
was read at 570 nm using ELISA reader.
with 20 mM ferric nitrate 30 mM H2O2 in
The experiments were done in triplicates.
20.0 mM phosphate buffer (pH 7.4) in a
Percentage cell growth was calculated as:
final reaction mixture volume of 20 μl for
Percentage cell viability = [At/Ac] ×100
1h at 37 oC. Oxidative DNA damage was
Percentage cell growth inhibition = (100-
induced by hydroxyl radicals generated in
percentage cell viability)
Fenton reaction (Ani et al., 2006). Bro-
Where At and Ac are absorbance of treat-
mophenol blue (0.25%) and glycerol
ed and control cells, respectively.
(30%) were added to terminate reaction
mixture, followed by gel electrophoresis
2.11. DNA content and cell cycle phase
in 0.7% agarose. The gel was then visual-
distribution
ized and photographed on gel doc.
Human pancreatic (MiaPaca-2)
cells were seeded in 6-well culture plates
2.9. Cells culture
5
with cell density 2x10 cells/ml/well and
Pancreatic cell line (MiaPaca-2),
incubated for 24 hrs. After incubation, the
Lung cell line (A-549), Prostate cell line
cells were treated with PDEE (0, 30, 50
(PC-3), Leukaemia cell line (THP-1), Co-
and 100 mg/ml) and again incubated for
lon cell line (HCT-116) and Lung cell line
48 hrs. After 48 hrs treatment cells were
(HOP-62) were purchased from National
collected by 5 min centrifugation at 1000
Cancer Institute, U.S.A and European
rpm. The harvested cells were washed
collection of cell culture, UK. Cells were
twice with phosphate buffer solution and
cultured in RPMI-1640 and MEM medi-
fixed with 70 % ethanol at -20 ºC for 1h.
um supplemented with nutrients and anti-
The cells were then stained with DNA
biotics. Cells were grown at 37 oC and 5%
staining solution containing propidium
CO2 level with relative humidity of 98%.
iodide (20 mg/ml) and triton X-100 (1%)
in PBS for 30 min in dark. FACScan was
2.10. Anticancer activity
used to measure DNA content. For each
The anticancer activity of PDEE
data file, data was collected from 10,000
against different human cancer cell lines
cells. Cell Quest (Becton, USA) was used
was evaluated by SRB assay as described
for analysis of histograms.
by Monks et al., 1991. Briefly 100µl of
cell suspension (1x105 cells/well) were
2.12. Loss of Mitochondrial Membrane
cultured in 96-well plates and incubated
Potential (ΛΨm)
overnight at 37 0C and 5% CO2 level. 20
Flow cytometry was used to meas-
µl test material at various final concentra-
ure the mitochondrial membrane potential
tions (10-100 µg/ml) was added. Paclitax-
loss (ΛΨm). Human pancreatic (MiaPaca-
el (1 µM) and 5-fluorouracil (20 µM)
2) cells were plated in 6-well cultural
were used as standard anticancer drugs.
6
plates with cell density of 1x10
Cell growth was stopped after 48 hrs of
cells/ml/well and incubated for 24 hrs at 5
incubation by adding 50 μl of 50% TCA
% CO2 level. The cells were then treated
in each well and incubated further for 1h
with PDEE (0, 30, 50 and 100 mg/ml) and
at 4 0C. The plates were then washed, air
again incubated for 48 hrs. Rhodamine-
dried and stained with 50 μl of 0.4% SRB
123, a cell permeable cationic dye was
dye in 1% acetic acid, followed by incu-
added one hour before termination of ex-
bation for 30 min at room temperature.
periment and again incubated for 30 min.
The unbound dye was then removed by
The cells were washed with PBS and pel-
washing with 1% acetic acid and kept
lets were collected by centrifugation. The
overnight for drying. In each well, 100 μl
collected pellets were re-suspended in 300
of 10 mM tris-base was added to solubil-
ml of PBS. Florescence of Rh-123 in cells
ise the dye followed by stirring for 5 min
ISBN: 978-967-14475-3-6; eISBN: 978-967-14475-2-9 516
Biotech Sustainability (2017)
Free Radical Scavenging Potential and Anticancer Activity of Primula sp. Wani et al.
were analysed at 485 nm by flow cytome-
them to act as strong reducing agents, hy-
ter (Jung et al., 2006).
drogen donor’s metal chelaters and singlet
oxygen quenchers (Miguel, 2010). The
2.13. Statistical analysis
total flavonoid content in DPEE was
All of the experiments were done in
found to be 7.06 ± 1.31 (mg catechin/g
triplicate. The data were recorded as
dry extract). Flavonoids are known exhib-
means ± standard deviations and were
it many biological activities like antioxi-
analysed with SPSS software.
dant, anticancer, antimicrobial and anti-
inflammatory properties (Hodek et al.,
3. Results and discussion
2002).
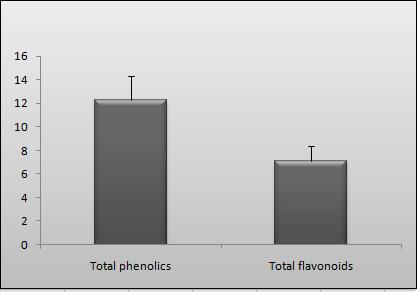
3.1. Total phenolic and flavonoid content
3.2. DPPH free radical scavenging activi-
Polyphenolic compounds are very
ty
important plant bioactive constituents be-
DPPH is a purple stable free radi-
cause of their scavenging potential due to
cal at room temperature with characteris-
the presence of hydroxyl groups (Hatano
tic absorbance at 517 nm. The nitrogen
et al., 1989). Folin-Ciocalteu method is
free radical of DPPH is easily quenched
most widely used to measure the poly-
by an antioxidant to yellow coloured
phenol contents, with the basic mecha-
complex
(1,1-diphenyl-2-picrylhydra-
nism of electron transfer and reducing
zine). The decolourization of purple col-
ability (Prior and Schaich, 2005). Using
our is stoichiometric depending on the
this quantitative assay, we found that the
number of electrons gained (Soares et al.,
total phenolic content (TPC) of ethanolic
1997; Mokbel et al., 2006; Singh et al.,
leaf extract of P. denticulate was found
2002). DPPH radical scavenging potential
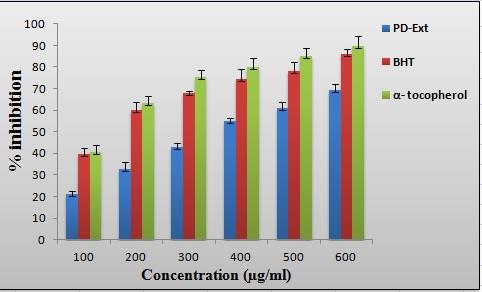
to be 12.24 ± 2.11 (mg GAE/g dry ex-
of PDEE at different concentrations in-
tract) as depicted in Figure 1. Considera-
vestigated in the present study was deter-
ble attention has been received by poly-
mined together with standard antioxidants
phenols for their physiological role as an-
(BHT and α-tocopherol) at the same con-
tioxidant and anticancer agents (Othman
centrations (Figure 2). PDEE showed sig-
et al., 2007). Polyphenolic compounds h-
nificant scavenging effect on DPPH free
Figure 1: Represents the total phenolic
and flavonoid content of PDEE. Each
value represents the mean ± SD (n = 3).











