Chapter 7
Understanding Atoms
All matter that we see is made of tiny particles called atoms. A long time ago, Democritus thought of an entity that was indivisible, he called it the final stage of all matter. He imagined that what would he get if he keeps on cutting an item with a very sharp knife? Will there be an ending stage? He stated that yes there is an end and he named this an atom.
So, according to Democritus, all things were made of tiny indivisible particles called atoms. According to him, one could not further divide atoms into smaller particles. That would be impossible according to him. John Dalton was an English school teacher whose theory of atoms was the first to leave an impact on the scientific community. He stated that different atoms represented different elements. He stated that atoms could neither be created, destroyed nor be transformed into other atoms. In a chemical reaction, according to him, there is only a rearrangement of atoms. Dalton’s theory assumed atoms to be indivisible and spherical, which we now reject.
Joseph Thomson, discovered electron, a sub-atomic particle contradicting Democritus and Dalton. His experiment was a consequence of photoelectric effect. He found that all things were made of atoms, but in turn, all atoms had a negative charged particle which he named electron. Not only that, Thomson also predicted the existence of a positive particle – since atoms are neutral as a whole. Proton, a positive particle was soon discovered. Proton has charge exactly equal to that on an electron but the polarity is opposite. An electron is negative, on the other hand, a proton is positive. Two sub-atomic particles were discovered and the name atom, which meant indivisible in Greek was contradicting the then theory.
Later experiments showed the possible existence of a third sub-atomic particle. Obviously this particle had to be electrically neutral otherwise it would make an atom charged up (an atom is neutral by birth)
Rutherford found that masses of atoms found experimentally were not agreeing with those calculated on paper. On paper, we were considering the masses of protons and electrons, yet we were very short of the masses found in spectrometer experiments.
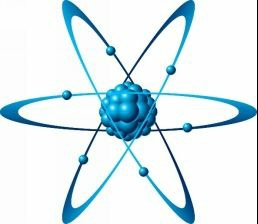
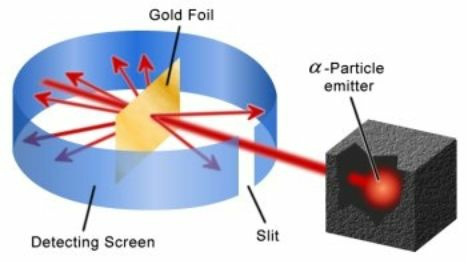
Rutherford modified Thomson’s theory by stating that there was another particle inside an atom. To illustrate this, he had designed an experiment. Locations of protons and electrons were not stated clearly in Thomson’s model of atom. Rutherford had planned to bombard high-speed alpha particles (by the way, alpha particle is a charged particle which has charge exactly double of that on a proton) on a very thin gold-foil. He was actually measuring interaction of alpha particle and gold atom. He found amazing stuff! The results were so astonishing that he had to totally change the Thomson’s model!
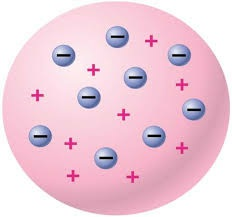
Thomson had created a model like that of a sphere – so he assumed atoms to be spheres, and he also assumed that all the mass of atom was uniformly distributed in this sphere – but Rutherford disproved his model.
Rutherford found that most of the alpha particles passed straight through the foil. This meant that most of the atom was empty space. If it were uniformly distributed with mass, the alpha should have rebounded, like a light particle hitting a mirror.
Another thing that Rutherford found was that a few particles deflected from their usual path at small angles, say 15 degrees or so. This meant, that somewhere inside the atom, a few locations were occupied by electrons, which were applying an electric force on alpha particles, causing them to deflect.
He found that one in 20000 alpha particles rebounded exactly at 180 degree angle. He assumed that the particle must have had hit a very dense solid. This was named nucleus. Rutherford stated in his atomic theory that most of the mass was located at extremely small and extremely dense area called the nucleus, located at the centre of the nucleus and that electrons were revolving around the centre just as planets revolve around the sun. Nucleus contained protons and neutrons, (the neutral particles) and electrons revolved around the nucleus.
There is one way so that you can experience Rutherford’s experiment. This experiment is discussed in the revision and I hope that you do this one, it is really interesting.
Now we will try to know how colours are made. The theory of atoms is not yet over. There is a lot of understanding that is left, you would be amazed but Rutherford’s model was falsified by Neils Bohr, whose model was again falsified by the collective efforts of Heisenberg and Schrodinger. You see science and especially physics is about checking and validating. No matter who you are, if one single experiment disagrees with your theory, your theory is falsified and no longer valid. For the sake of simplicity, we will discuss about colours in the simplest way possible, for a detailed and simplified explanation, you should also invest your time on one of these books:
1. Light, colours and the physics behind, this book is devoted to the physics of colours
2. Let us discover modern physics, if you want to know more about atomic theory by Bohr and Heisenberg’s modification in the simplest language possible
3. What is this world made of? A book which discusses atomic theories for the best of your understanding of this world and beyond
The main objective of all books is to enjoy physics, because physics is beyond equations, it is in experience.
So we were talking about colours. Colours are actually made by atoms. So imagine a yellow coloured wall. Why is it yellow? It is yellow because light falls on its surface and it reflects back only the frequency corresponding to yellow. When it is very dark in the room you are in, you do not see the wall. So you see the wall, when light falls upon it. According to Einstein, light is made of particles which he named photons, so when these photons fell on the wall, having some energy, they excite atoms of the wall. A neutral and not so excited atom has no absorbed energy. As we already know, when light falls on a rough surface, most of the incident energy is absorbed and only a particular frequency is reflected back, which we see as its colour. When light falls on a very smooth and very shiny surface, such as a mirror, almost all (sometimes 100%) the light is reflected back, thus you see your reflection, mirror does not have a colour because all the light that falls on it (which is white light of sun) is reflected back (which is again a white light)
So what we see as colours are actually frequencies, visible frequencies. So when light falls on the atoms of wall, these atoms get excited, an excited atom is the one which has absorbed energy. But atoms are not stable at all in their excited states. They want to get normal. How can nature make them normal? It is done by re-emission of the energy that they had absorbed. This re-emission is what we see as colour. In the dark, no light falls on the atoms of this wall, no excitation occurs and thus no colours are seen.
Maybe this seems confusing. But if you read the above two paragraphs again and very carefully, you will understand what I mean, but I strongly recommend Modern Physics or Light, colours and the physics behind. Your understanding about atoms and light would be much stronger than it is now!
Revision 3
1. What is light? How do stars make light?
2. How do you see your reflection on a mirror? How did Isaac Newton explain reflection?
3. What is refraction? Have you ever experienced refraction? Discuss about it.
4. What is sunlight made of? Take a chart paper and cut a circle. Colour the seven colours of the sunlight on this circle and stick this paper circle on a solid cardboard. Now you have made a circular disk. The colours are violet, indigo, blue, green, yellow, orange and red. Rotate this disk fast enough. What do you see? Why?
5. What was the argument between Newton and Huygens?
6. How did the double slit experiment falsify the particle theory of light and establish the wave model?
7. So what exactly is light? Is it a wave or a particle?
8. What is an atom? Is it indivisible as Democritus had assumed?
9. State the postulates of Dalton’s atomic theory. Why do you think John Dalton is considered as the father of modern atomic theory?
10. What is photoelectric effect? How did Thomson use this to predict the existence of a sub- atomic particle? Explain in detail.
11. Why did Rutherford suspect Thomson’s model of atom? What experiment did he suggest?
12. What were the results of Rutherford’s experiment with alpha particles? How did he completely change Thomson’s model of atom?
13. What is a nucleus?
14. Why should the number of protons and the number of electrons be the same in a neutral atom?
15. An atom has 10 protons and 13 electrons. Is it neutral? Express its charge equivalent to that on an electron.
16. What is an excited atom? Is an excited atom equally stable as a neutral atom?
17. Rutherford’s experiment can be replicated at home! Stand next to a wall. Draw a very small target on this wall, a target as big as a tennis ball. Now stand 50 metres away from this wall and aim tennis balls at it. Do you hit the target? Most of the time, you will fail – this same thing happened with Rutherford. 1 in 50 times, you may hit the target! Rutherford is proud of you…
18. How are colours produced? Why do you see green colour of grass during the day and not see it at all when it is totally dark?






