Chapter 5
Understanding Light
Without light, you cannot see. Try to find your way in dark, if you are familiar with the things around, you will do this with ease. But try to walk around in a dark and gloomy tunnel where there are numerous items scattered on the floor. You would hurt yourself. Darkness can be removed with lights of all colours. Most people are not aware of the fact that colours are also lights. In fact, light that we see is made of seven different colours and the image below tells you what I want to convey.

Just like our ancestors wanted to understand motion and gravity, they were also keen about the nature of light. They asked numerous curious questions: What is light? What is it made of? How fast does it travel? Where does it come from? What is its nature? Only a curious and inquisitive mind can ask such questions. Take for example Carl Sagan, who was once puzzled by the stars. He asked, what were they? The most usual answer that he got was ‘They are lights in the sky, kid.’ The correct and somewhat more satisfying answer that he found was that stars were distant suns. This allowed him to wonder at the grand design of the universe and left his young impressionable mind in joy.
So when kids ask questions, they are trying to know of something – in fact they may be curious to learn something new. We, as adults should promote their desire and not suppress it with usual and boring answers. With kids, adults often become smarter. So what will you tell your kid if he asks you ‘How does a bulb glow?’ You tell him (or her) about conversion of energy. What is he asks you about light? So let us understand what light actually is:
It was Isaac Newton (yes, again!) who first questioned about the nature of light – he compared light as a stream of particles. He believed in his particulate model of light. Just as a pen or pencil or any other object is made of small particles called atoms – Newton assumed that light, too, was composed of small microscopic particles that carried light energy.

In this way, Newton could explain reflection – the way you see yourself on the mirror. You see your reflection because light particles move and collide with shiny surface of mirror and bounce back. Compare these particles with bouncing balls. You aim a ball at a wall and it bounces back at you. Light is thought of as small balls bouncing back at you. But Newton explained that the reflection is not always perfect. For example, you can also see your reflection on the back side of a spoon but it is not as clear as what you see on the mirror. This is because rough surfaces as explained by Newton would always absorb most of the light particles. Those surfaces that do not absorb but always allow the light particles to bounce back produce the best reflection – like that mirror next to you.
But Newtonian view of light was challenged. Newton could not explain refraction. He did but his explanation was vague. Refraction is very different from reflection. While reflection is bouncing back of light rays, refraction involves bending of light rays. You may have experienced refraction when you are in a swimming pool. You appear to be shortened. The picture below clearly illustrates what refraction is – it is the bending of light when light changes media (such as from air to water) and Newtonian theory of gravity was not fit to explain bending.

A new idea was waiting for approval – that light was a wave that carried light energy. This idea was put forward by Christian Huygens who stated that light travelled as a wave. He stated that wave travels with some speed and when light enters from one medium to another, its speed changes, thus bending occurs. But again, Huygens theory could not perfectly explain reflection. Actually the idea is: Both theories could explain some aspects of light but both theories also failed at certain light phenomena. Scientists were confused about what was correct and what was wrong. The major belief was that light couldn’t be both!
Newton’s theory said that if you shine a torch at a wall and shine another at the same wall, you make a brighter light on the wall – the wall lights up! But there was a contradictory phenomenon that scientists discovered, including Thomas Young. He found that when a light ray from a similar source passed through two very narrow slits – as thin as a thread, there were patterns of bright and dark patches. He expected only brightness – as he believed in Newton but what he saw amazed his eyes! He found that light plus light could also create darkness! So in our analogy, it is like you shine the two torches at the wall, and you see no brightness, nothing but darkness! The diagram below shows you the pattern as seen in the two-slit experiment.
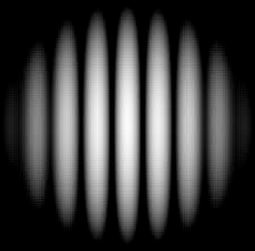
Scientists all around the world started to discard particle theory because it did not predict such a thing! They started analyzing the wave model of light and found a possible explanation. Below you see a wave, it has ups and downs as you see in a water wave.
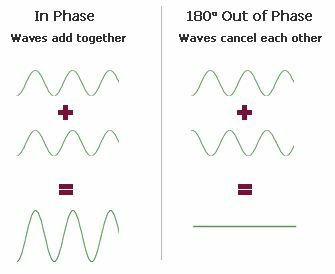
If you mix two waves you can create two patterns: One of them is called constructive mixing and the other is termed destructive mixing. If you look at a wave, you can create these two patterns. When two waves mix at the same level, they produce light – light + light = much brighter light but when you mix two waves differently – at different levels, you create a dark patch – light + light = darkness. Just look at the diagram below to understand this again.
It is like addition. You add 2 and 2, only to get 4. But if you add 2 with (-2) you get nothing. This is what happens with light waves. Same levels mean same signed waves and different levels mean waves of opposite phases. This is pure analogy.
But the story is not finished. Particle model wanted revenge, wave theory had overthrown its power when Young did double-slit experiment but when Hertz performed photoelectric experiment, only particle model of light could explain what was happening.
Heinrich Rudolf Hertz, a German experimental physicist found that when light rays of suitable (required) energy hit a metal surface, electrons were emitted out of the metal – like a current, when light (photo) hit metal producing current – thus the name photoelectric, relating light and electrons. Hertz also found that when a negative charged metal plate was placed under incident light of required energy, the negative charge on the metal decreased. Also, when a positive plate was kept, it got more and more positive. This led Joseph Thomson, an English physicist, to assume that metal atoms, in fact all atoms, had tiny particles that carried negative charges. He explained that when light of suitable energy fell on metal like sodium or calcium, electrons (which was the name of these negative particles) were emitted out with some kinetic energy.
So what was happening? Light energy was changing to kinetic energy of electrons. At first, it seemed that wave theory would easily be able to explain this phenomenon, and that particle model would not be needed. But as it turned out, wave theory failed. We would not further discuss of what happened – but one thing was clear. Light was of mysterious character – it behaved like wave at times (light + light = darkness) and like particle at times, to explain emission of electrons from metal (in which light particle transferred their momenta to electrons to give these negative charges some kinetic energy). If you want to know more about photoelectric effect and about its explanation, you should check out this book: Light, colours and the physics behind or Let us discover modern physics in which you learn about wave-particle duality.

Now let us understand something about what colours are. You see a variety of colours around you. You see red, green, yellow, blue and so many colours, that it is almost impossible to list them all. But what are they?
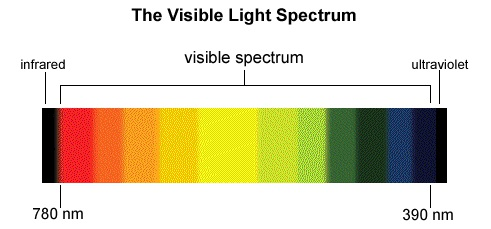
Actually a colour is a light of special nature. To each colour, a special property is associated – this is called frequency. Violet or blue is the most frequent light. Red and orange are less frequent light waves. It is best to understand colours as waves. So when you see red colour, you are actually looking at a particular frequency of light, a slight increment or decrement from the red frequency lets you create a new colour. This is used by computers that can create millions of colours by varying frequency of standard colours. The sunlight that you see is composed of seven different colours. They mix so well that sunlight appears to be white. You can see this for yourself, take a CD, and let sunlight fall on it. Do you see different colours? These arise from the sunlight as sunlight is composed of them. Why do we see colours? This question is well answered in the next chapter, called Understanding Atoms.










