Chapter Two
2. IMAGING TRANSMEMBRANE PROTEIN
TRANSPORT ACROSS THE NUCLEAR
ENVELOPE
Mark Tingey, Steven J. Schnell, Yichen Li, Samuel Junod, Wenlan Yu, Weidong Yang*
Department of Biology, Temple University, Philadelphia, Pennsylvania, USA
ABSTRACT
The mechanism of membrane protein transport across the nuclear envelope remains a point of discussion among researchers resulting in four proposed models: diffusionretention, ATP-dependent, nuclear localization signal–mediated, sorting motif– mediated. Confirming any of these models is hampered by the challenges of resolving proteins on the outer nuclear membrane and the inner nuclear membrane. Whilst differentiation via imaging is possible using immunogold-labeled electron microscopy as well as various super-resolution methods, typically these methods require the sample to be fixed or frozen, and provide limited information regarding the dynamic transport mechanisms of nuclear membrane proteins. Recently a number of dynamic single-molecule microscopy techniques have been developed and utilized to elucidate the transport mechanism of membrane proteins in our laboratory. Here we evaluate the existing evidence for each model, the techniques used to obtain that evidence, and ways in which single-molecule microscopy can be used to further interrogate the question of nuclear envelope transmembrane protein transport.
Keywords: single-molecule microscopy, SPEED, NETs, transmembrane protein, nuclear envelope, transport, single-molecule FRAP, HiLo
* Direct all correspondence to Dr. Weidong Yang, Department of Biology, Temple University, Philadelphia, Pennsylvania. E-mail: Weidong.Yang@temple.edu
2.1. INTRODUCTION
The nuclear membrane of eukaryotic cells consists of two lipid bilayers, the inner nuclear membrane (INM) and the outer nuclear membrane (ONM). The INM and ONM are sperated by a perinuclear space of approximately 30-50 nm (Franke et al., 1981; Feldherr and Akin, 1990). Together, these structures form the nuclear envelope (NE), which functions as a barrier between the nucleus and the cytoplasm of the cell. Embedded within the INM and ONM are nuclear envelope transmembrane proteins (NETs). Each membrane contains a unique set of NETs that are targeted to their specific compartments after synthesis at the endoplasmic reticulum (ER). The ER is contiguous with the ONM and fuses with the INM where nuclear pore complexes (NPCs) are inserted (Gerace and Burke, 1988). The NPC is a large complex composed of more than 30 nucleoporin (Nup) proteins that function together to regulate the transport of proteins and RNA. The primary pathway for soluble proteins and RNA to pass through the NE is the central channel of the NPC. The central channel has a minimum diameter of approximately 50 nm and functions as a selectively permeable barrier against passive diffusion of large molecules (Beck et al., 2004; Lim, Fahrenkrog and Köser, 2007). The barrier in the central channel is formed by intrinsically disordered phenylalanine-glycine (FG) motifs on one third of the Nups (Frey et al., 2007). In addition to the central channel, the NPC also contains multiple peripheral channels, which are structures approximately 10 nm wide between the core protein structure of the NPC and the membrane (Reichelt et al., 1990; Hinshaw, Carragher and Milligan, 1992). These peripheral channels remain poorly described; however, they have been implicated in the transport of NETs from the ONM to the INM.
The transport of NETs to their appropriate locations on the ONM and INM is critical to the proper function of the cell. ONM and INM NETs have been shown to play a role in genome function as well as providing structure to their respective membranes. For instance, a variety of INM NETs bind the lamina intermediate filament network associated with the INM (Hetzer and Wente, 2009; Arib and Akhtar, 2011; de Las Heras, 2013). Furthermore, ONM NETs connect to all three major cytoplasmic filament systems as well as forming connections across the lumen of the NE to form the nucleoskeleton and cytoskeleton (LINC) complex critical to mechanosignal transduction based regulation of the genome as well as cell and nuclear mechanical stability (Crisp et al., 2006; Zuleger, Korfali and Schirmer, 2008; Östlund et al., 2009). Loss of function in these complex interactomes can result in a variety of pathologies, often termed envelopathies or laminopathies. These pathologies are often highly tissue-specific, thereby highlighting the importance of INM protein function during development (Schirmer and Gerace, 2005; Chi, Chen and Jeang, 2009).
The topography of individual NETs also provides insight into the putative function and interactions between NETs and surrounding proteins. The KASH (Klarsicht, ANC-1, Syne homology); SUN (Sad1p, UNC-84); and LEM (laminassociated protein [LAP]2, Emerin, MAN1) domains represent families of NETs with functions critical to proper cell function. The LEM domain is a globular approximately 40-residue helix-loop-helix motif found primarily in several unrelated INM NETs (Lin et al., 2000), including MAN1, Emerin, LEM2, and several alternatively spliced isoforms of LAP2 (Wagner and Krohne, 2007). Proteins containing the LEM domain directly interact with the highly conserved barrier-to-autointegration factor (BAF) protein, a DNA bridging protein. Furthermore, all LEM-domain containing proteins bind directly to A- or B-type lamins in vitro (Gruenbaum et al., 2005). LEM domain NETs have been implicated in many critical genome regulation processes, including regulation of transcription factors, signal transduction, and chromatin structure (Barton, Soshnev and Geyer, 2015).
The KASH domain is a highly hydrophobic domain approximately 60 residues long, consisting of an approximately 20-residue transmembrane region and an approximately 35-residue C-terminal region that lies between the INM and ONM (Wang et al., 2012). The KASH domain interacts with the SUN domain, a specialized form of the discoidin domain found in INM NETs (Mans et al., 2014). The interaction between the KASH domain and the SUN domain forms the LINC complex and mediates subsequent nuclear movement and positioning (Crisp et al., 2006; Cain et al., 2018).
The localization of INM-NETs to their proper regions is critical to proper cell function; therefore, the mechanism by which NETs translocate from the ER to the INM after synthesis is an area of interest to researchers. The exact mechanism of translocation remains a subject of debate, and four prevailing hypotheses will be discussed in this chapter. These models of the transport of NETs from the ER and ONM to the INM share a similar basic pathway; specifically, proteins are produced in the ER, then travel across the ONM and through the NPC to the INM. The key difference in the models lies in the associated proteins and requirements for this passage. Although there are some reports of NETs translocating to the INM in a manner bypassing the NPC (Dixon and Schirmer, 2018), this chapter focuses on the transport models of NETs passing through the NPC.
2.2. PROPOSED MODELS FOR NUCLEAR ENVELOPE TRANSMEMBRANE PROTEIN TRANSPORT
2.2.1. Diffusion-Retention Model
The diffusion-retention model is also referred to as the Lateral diffusion retention model and was the uncontested model for NET transport across the NE for many years. The diffusion-retention model was first proposed following a pair of discoveries, the first being identification of an approximately 10-nm channel peripheral to the central channel of the NPC (Hinshaw, Carragher and Milligan, 1992), which pointed to the potential existence of transport channels beyond the canonical soluble protein transport routes through the central channel of the NPC. The second discovery was the observation that the small INM protein p55 is capable of translocation between nuclei in a fused cell (Powell and Burke, 1990).
These two discoveries make up the basis for the diffusion-retention model, The core idea being that proteins freely and rapidly diffuse between the ER, ONM, and INM. The model further postulates that the mechanism behind aggregation of INM proteins on the INM is due to lamins and peripheral chromatin binding to INM NETs (Figure 1).
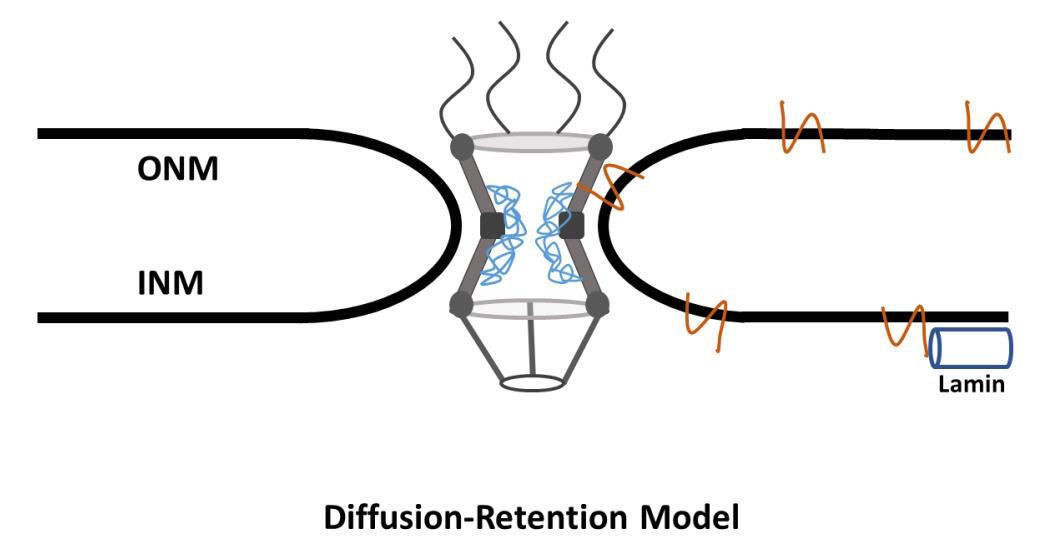
Figure 1. Diffusion-Retention Model of NETs Transport. NETs of a size ~70 kDa are synthesized at the ER. They then passively diffuse across the ONM, through the peripheral channel, and onto the INM. If they interact with a nuclear protein, i.e. lamin, they will be retained in the nucleus. If they do not interact with a nuclear protein, they will passivly diffuse from the INM to the ONM.
In the diffusion-retention model, translocation of a transmembrane protein begins at the ER. The transmembrane protein is either co-translationally or posttranslationally inserted into the membrane of the ER, likely in a Sec61-dependent manner (Rapoport et al., 2004; Park and Rapoport, 2012; Katta, Smoyer and Jasperson 2014). Because the ER is contiguous to the ONM (Amar-Costesec et al., 1974), the protein is can freely diffuse from the ER to the ONM. Then, according to the diffusion-retention model, the protein diffuses through the peripheral channel of the NPC into the INM where it will bind to lamins and peripheral chromatin. If the protein does not have the required binding sites, it will then diffuse back through the peripheral channel to the ONM and ER (Katta, Smoyer and Jasperson, 2014; Dixon and Schirmer, 2018).
The supposition that INM proteins aggregate in the nucleus due to interactions between the NETs and proteins within the nucleus is supported experimentally by the observation that proteins that do not typically enrich at the INM will localize to the INM if a binding domain specific to nuclear proteins is added. Soullam and Worman first demonstrated this phenomenon by altering the transmembrane region of an ER-specific protein to express the lamin-binding region of an INM-specific NET, which subsequently localized to the INM (Soullam and Worman, 1993). This observation was further validated using other ER-specific proteins and a variety of INM NET-specific lamin-binding regions, providing further support for this model (Furukawa , Fritze and Gerace; Wu, Lin and Worman, 2002; Zuleger, Korfali and Schirmer, 2008).
A number of studies have assayed NETs for binding affinity for nuclear localized proteins and have shown that the vast majority of NETs directed to the INM exhibit binding affinity for chromatin or chromatin-associated proteins (Dixon and Schirmer, 2018). A good example of this affinity is the LEM domain family of NETs, which all contain a motif facilitating binding to the chromatin associated BAF protein, thereby enabling LEM domain NETs to aggregate on the INM (Brachner et al., 2005). Researchers have also made use of fluorescence recovery after photobleaching (FRAP) microscopy to determine the proportion of tagged mobile INM NETs as a method of assaying the diffusion-retention model. When FRAP of the fluorescently tagged INM-based Lamin B Receptor (LBR) was performed, it was shown that 60% of prephotobleach fluorescence failed to recover after photobleaching (Ellenberg et al., 1997). Approximately 60% of LBR on the INM is non-mobile and unable to diffuse. This observation strongly supports the assertion that proteins diffuse into the INM from the ONM, interact with proteins within the INM, and become immobile. Furthermore, numerous studies have shown a decrease in the mobility of NETs on the NE when compared to the ER, which suggests that the binding affinity in assayed NETs for nuclear proteins inhibits mobility (Ostland et al., 1999; Shimi et al., 2004; Zuleger et al., 2011).
The approximately 10-nm peripheral channel is sufficient to allow membrane proteins to diffuse through the NE in a manner consistent with the measured diffusion rate, provided they have a nucleoplasmic or extraluminal domain approximately 40 kDa or smaller (Pain et al., 1975; Reichelt et al., 1990; Hinshaw, Carragher and Milligan, 1992; Maimon et al., 2012). The peripheral channel has been further implicated in NET transport to the INM in an experiment that blocked the peripheral channel. Researchers introduced a nuclear pore glycoprotein-210 (gp210) antibody, which binds to gp210. This gp210 antibody anchors the NPC to the membrane and is located near the peripheral channel (Greber, Senior and Gerace, 1990). In the presence of a gp210-specific antibody, the peripheral channel is occluded. Blocking the peripheral channel with the gp210-specific antibody severely impaired the ability of INM NETs to translocate to the INM (Ohba et al., 2004), further confirming that INM NETs require the peripheral channel for entry into the INM.
The diffusion-retention model is an energy independent model that relies on passive diffusion and is therefore limited by the size of the pheripheral channel. This limit has been confirmed in multiple studies that observed slowed transport as protein size increased or an inability to translocate if the protein became too large. Chimeric proteins of approximately 60 kDa and larger have been observed in multiple experiments to be unable to pass through the NPC into the INM, further supporting the size limitation proposed by the diffusion-retention model (Soullam and Worman, 1993; Soullam and Worman, 1995; Wu, Lin and Worman, 2002; Ohba et al., 2004; Boni et al. 2015; Ungricht and Kutay, 2015). For many years, the diffusion-retention model was mostly uncontested as the de facto model of NET transportation; however, many exceptions to this model have been observed, which suggests that the process is perhaps much more complex than is represented by this model.
2.2.2. ATP-Dependent Model
The diffusion-retention model of INM transport revolves around the concept of energy independence. Diffusion is a passive process that does not require adenosine triphosphate (ATP) to accomplish. To interrogate whether ATP is required for NETs to translocate to the INM, Ohba and colleagues developed a rapamycin trap assay that would enable them to assay whether or not ATP was required to transport NETs into the INM. This study used LAP2β, a type II integral protein of the INM, as a reporter. This reporter was then modified to have the FRB (FKBP12/rapamycin-binding) domain of mTOR (mechanistic targeting of rapamycin) fused to the N-terminus and GFP fused to the C-terminus. The second component of this trap was a nuclear localized protein containing three repeats of FKBP12. In the presence of rapamycin, FRB and FKBP12 will bind, enabling researchers to determine if the reporter protein was successfully translocated to the INM (Ohba et al., 2004).
At reduced temperature and in the absence of ATP, translocation of the reporter to the INM was completely inhibited. However, reporters present on the ONM and ER were unaffected. This strongly suggests that ATP is required for translocation of NETs to the INM. However, it must also be noted that this experiment was performed with artificial markers, which may have also impacted translocation of NETs (Ohba et al., 2004).
The observations made by Ohba and colleagues led Zuleger, Korfali, and Schirmer to suggest a possible new transport model, initially dubbed gated lateral diffusion, wherein the authors suggest that Nup gp210 functions as a gatekeeper for the peripheral channel. This initial model proposed a mechanism in which proteins would be required to pay a fee of ATP to gp210, causing a conformational change allowing the proteins to then pass through the NPC into the INM (Zuleger, Korfali and Schirmer, 2008).
This idea was further refined in a subsequent study that also provided experimental support for the ATP-dependent model. In this study, authors assayed six proteins for their dependence upon ATP to translocate to the INM. Of the six proteins assayed, only emerin and SUN2 were found to require ATP to translocate to the INM (Zuleger et al., 2011). SUN2 and emerin exhibited decreased mobility on the ER as well as the ONM in the absence of ATP. This change suggests that ATP is not required as a fee to cause conformational changes in gp210; rather, ATP is required for a change to the protein prior to transport through the NPC. The ATP-dependent model begins with protein synthesis at the ER, where it is suggested that a chaperone or ER retention protein may be bound to the NETs. ATP is then required to release the NETs and allow translocation to the INM (Dixon and Schirmer, 2018). This model is still in its infancy, with little direct experimental evidence; however, as the most recently proposed model, this is to be expected (Figure 2).
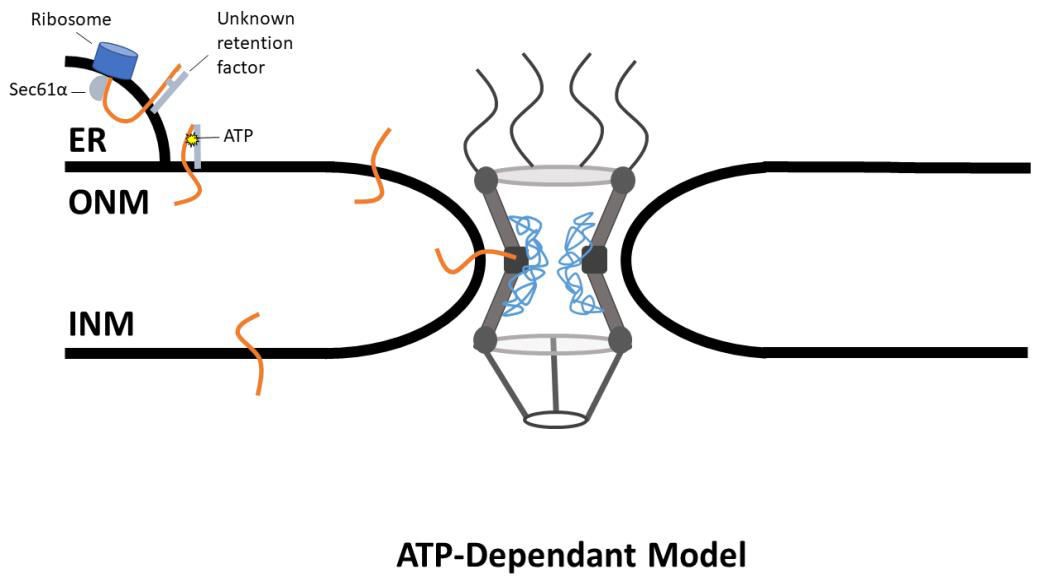
Figure 2. ATP-Dependant Model of NETs Transport. NETs are translated at the ER, where Sec61α inserts the protein into the membrane. The NET is then bound to an unknown retention factor, preventing the NET from diffusing across the NE. The bond between the NET and the retention factor is broken in an ATP dependent manner, and the NET then diffuses across the NE through the peripheral channel.
2.2.3. Nuclear Localization Signal–Mediated Model
Soluble protein transport into the nucleus, in a simplified model, takes place in three stages. First, the nuclear localization signal (NLS; a small <50 amino acid sequence) of a cargo protein is recognized by karyopherin-α/β. Second, the complex of the cargo and the karyopherin pass through the NPC by interacting with a subset of Nups enriched with FG repeats. Third, karyopherins release the cargo when stimulated by the small GTPase Ran in its GTP-bound form (Fried and Kutay, 2003; Lusk, Blobel and King, 2007).
In their 1995 paper, Soullam and Worman identified a signal in the N-terminal nucleoplasmic domain of chicken LBR that functioned similar to a canonical NLS. This indicated that there was, on some level, crossover between the membrane-bound and soluble protein transport pathways; however, the idea that the NLS may be critical for INM targeting was largely discarded; chimeric proteins containing NLS sequences were unable to translocate into the INM (Soullam and Worman, 1995).
This concept may have been discarded too soon. Ten years after Soullam and Worman published their findings, King and colleagues showed transport in budding yeast of the membrane-bound LEM domain–containing proteins Heh1/Src1 and Heh2 relied upon karyopherins and the Ran GTPase cycle to aggregate in the INM. Furthermore, King and colleagues identified a sequence akin to the canonical NLS on Heh2 that binds to karyopherins and is required for transport into the INM. When the putative NLS was deleted from Heh2, the YFPtagged proteins dispersed across the ER and ONM but were almost entirely excluded from the INM (King, Blobel and Lusk, 2006). Further analysis of the extralumenal domains of membrane proteins identified many putative NLS sites, suggesting that the phenomenon may not be isolated to Heh2, but is in fact widespread (Lusk, Bloble and King ,2007; Malik, Zuleger and Schirmer, 2009).
Observations made by Lusk, Blobel and King led them to publish “Rules for the Road”, a manuscript detailing a new transport model dubbed the “Transport Factor–Mediated” model. This model marries the diffusion-retention and the transport of soluble proteins models: a NET is synthesized at the ER and inserted into the membrane, then a karyopherin binds to an NLS in the extraluminal domain of the NET. The karyopherin then transports the NET through the NPC, where the karyopherin detaches in a RAN GTPase–dependent fashion (Lusk, Blobel and King, 2007; Katta, Smoyer and Jasperson, 2014) (Figure 3).
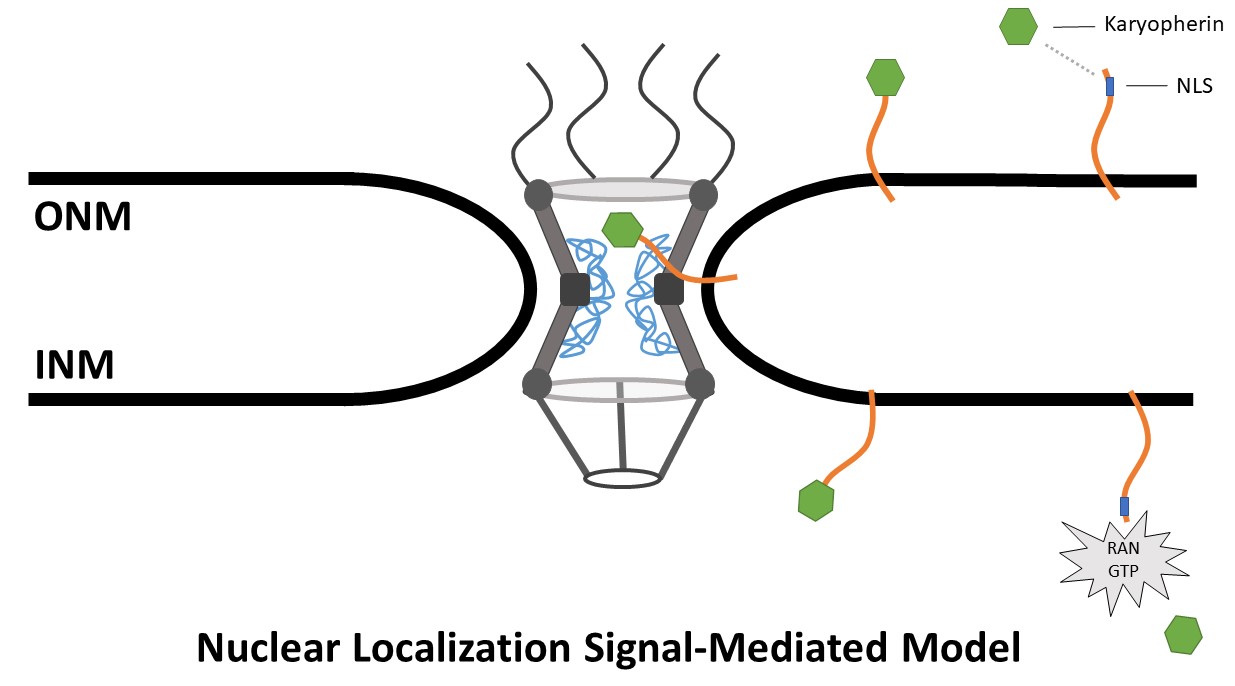
Figure 3. Nuclear localization signal-mediated model. NETs containing an NLS bind a karyopherin. The karyopherin then transports the NLS-containing terminus through the central channel of the NPC while the membrane bound terminus passes through the peripheral channel. The karyophern then disassociates from the NET in a RAN GTPase-dependent fashion.
In addition to proposing a new transport model, Lusk and colleagues codefied a series of rules for INM transport of NETs. These rules effectively amended the diffusion-retention model to have a lower size limitation for diffusion. The model states that proteins approximately 25 kDa or smaller can freely diffuse through the ER and ONM to the INM. These proteins may then aggregate in the INM due to interactions with other proteins within the nucleus. The size limitation is significantly smaller than the approximately 70 kDa limit of the diffusion-retention model, and excludes a large number of NETs from translocation via the diffusion-retention model.
To account for the larger proteins, the model states that NETs with an extraluminal domain between approximately 25 and 75 kDa require the assistance of an NLS to pass through the NPC. Furthermore, the strength of the NLS determines the specificity of the localization. For instance, a weak NLS would be enough to target a protein to the INM, but not to confer exclusive localization to the INM, whereas a strong NLS would target to a protein to the INM as well as confer exclusive localization to the INM.
This model raises the question that if NETs are making use of karyopherins to transport into the INM, do they also use the central channel of the NPC? A karyopherin bound to the extraluminal domain of a large NET is difficult to imagine passing through the peripheral channel of the NPC. To answer this question, Meinema and colleagues designed a rather elegant experiment to interrogate this question, wherein a synthetic INM NET was generated that fused the Heh2 NLS, linker and transmembrane region to the human protein FKBP12 tagged with eGFP. This protein then was expressed within a line containing a modified Nup Nsp1 fused to FRB. In the presence of rapamycin, FKBP12 and FRB will bind; therefore, if the synthetic Heh2 passes through the central channel of the NPC in the presence of rapamycin, it will bind to the FRB fused to Nsp1. If the synthetic NETs pass through the peripheral channel rather than the central pore, the FRB and FKBP12 will not bind. In addition to this experiment, combinations of FG Nups also were deleted. It was observed that the synthetic Heh2 aggregated in a manner consistent with central pore transport (Meinema et al., 2011).
Perhaps the most interesting conclusion drawn by Meinema and colleagues is the observation that a disordered linker is required for the NETs to make use of the central pore. They examined linkers of different lengths and compositions and determined that a linker consisting of at least 120 residues was required to facilitate transport. This indicates that the linker connects the transmembrane domain with the region that is interacting with the FG Nups, whereas the disordered linker slices through the NPC scaffold in an as of yet uncharacterized manner (Meinema et al., 2011).
The assertion that NETs associate with karyopherins to facilitate nuclear transport was further supported by the observation that the deletion of different combinations of FG Nups directly impacted the efficacy by which the synthetic Heh2 was able to translocate to the INM (Meinema et al., 2011). Together, these data make a very convincing case for the Nuclear Localization Signal–Mediated model.
2.2.4. Sorting Motif–Mediated Model
The NLS plays a large role in the translocation of NETs to the INM; however, not all proteins containing an NLS appear to rely upon that NLS for translocation to the INM. In fact, the deletion or mutation of the NLS has no impact upon NET translocation to the INM (Turgay et al., 2010; Tapley, Ly and Starr, 2011). The lack of effect of the deletion of the NLS on these NETs implies that another factor is targeting these proteins to the INM.
The bacculovirus Occlusion Derived Virus (ODV) follows a rather interesting infection methodology. The virus inserts into the ER and then migrates into the INM, where it forms a viral envelope. While investigating the transport route followed by this virus, it was discovered that the capsid protein ODV-E66 is directed to the INM by a short sequence of amino acids. This sequence, termed the inner nuclear membrane sorting motif (INM-SM), has two prominent characteristics: an extremely hydrophobic region of 18 amino acids and a number of positively charged amino acids close to the C-terminus of the hydrophobic region. Together with these features, a 33–amino acid sequence on the N-terminus of ODV-E66 was sufficient to traffic proteins to the INM (Braunagel et al., 2004).
The discovery of the transmembrane sequence (TMS) in viral proteins raised a very important question: Is there a sequence in host cells that perform a similar function? It was shown by Braunagel and colleagues that a sorting motif similar to that found in ODV-E66 is present in well-identified host INM NETs, including some LEM SUN domain– and SUN domain–containing proteins (Braunagel et al., 2004). On more thorough inspection, it was shown that both the viral and host INM-SM–containing proteins interact with the translocon in a similar fashion. However, they do interact with the translocon in a fashion distinct from other membrane proteins (Saskena et al., 2004).
Further investigation of the translocon showed that the INM-SM of both ODV-E66 and LBR interact with truncated membrane-bound karyopherin-α importin-α-16 (Braunegal et al., 2004, Saskena et al., 2004). The truncate lacks the importin-β binding site that enables keryopherin-α to interact with keryopherin-β to form the dimeric karyopherin complex. This observation has led investigators to propose that importin-α-16 functions independently of the full length karyopherin-α (Rexach, 2006). Given the smaller size of importin-α-16, it is likely that it is able to transport through the peripheral channel of the NPC while associated with its cargo (Rexach 2006; Saskena et al., 2006).
An interesting feature of importin-α-16 is the presence of armadillo repeat motifs (ARMs), which interact with FG Nups. Whilst the majority of FG Nups are localized to the central channel of the NPC, several Nups containing FG repeats are closely localized to the peripheral channel of the NPC (Batrakou, Kerr and Schirmer, 2009; Kerr and Schirmer, 2009; von Appen and Beck, 2016; Kosinski et al., 2016). The potential interaction between the ARM repeats on importin-α-16 and the FG repeat–containing Nups in the peripheral channel has caused some researchers to propose that importin-α-16 negotiates the FG Nups at the peripheral channel in a manner similar to how the importin complex negotiates the FG Nups in the central channel, provided the FG Nups are indeed accessible in the peripheral channel (Dixon and Schirmer, 2018).
In addition to interacting with membrane proteins, it was shown that importin-α-16 interacts with Sec61α. This information together forms the basis for a model in which INM proteins are recognized by Sec61α upon synthesis and then transported through the NPC by importin-α-16 to the INM, where release of importin-α-16 is then stimulated by Nup50 or some other Ran-independent mechanism (Gilchrist and Rexach, 2003; Braunegal et al., 2004; Matsuura and Stewart, 2005; Rexach, 2006; Braunegal, Cox and Summers, 2009) (Figure 4).
In an odd twist, a study of Heh2 trafficking in yeast (which was initially used to develop the Nuclear Localization Signal–mediated model) has identified an INM-SM sequence. The sequence interacts with truncated forms of Kap60 (Importin-α), Kap60-30, and Kap60-40, which were subsequently shown to be necessary for INM localization of Heh2 (Liu et al., 2010). Furthermore, mutating a previously unidentified INM-SM sequence decreased the localization of Heh2 at the INM in yeast. Interestingly, deletion of the INM-SM still resulted in Heh2 localizing to the INM (Meinema et al., 2011). This incongruous result has led some researchers to argue that in Heh2 yeast, the INM-SM may be used to determine membrane topology (Laba, Steen and Veenhoff, 2014).
Considering this evidence, it has been proposed that some INM-SM– containing proteins have redundant systems of transport into the INM. The contrasting view remains that the sorting motif–mediated model is a two-step process, wherein the INM-SM is recognized by Sec61α during translation and thereby targeted to the INM (Dixon and Schirmer, 2018).
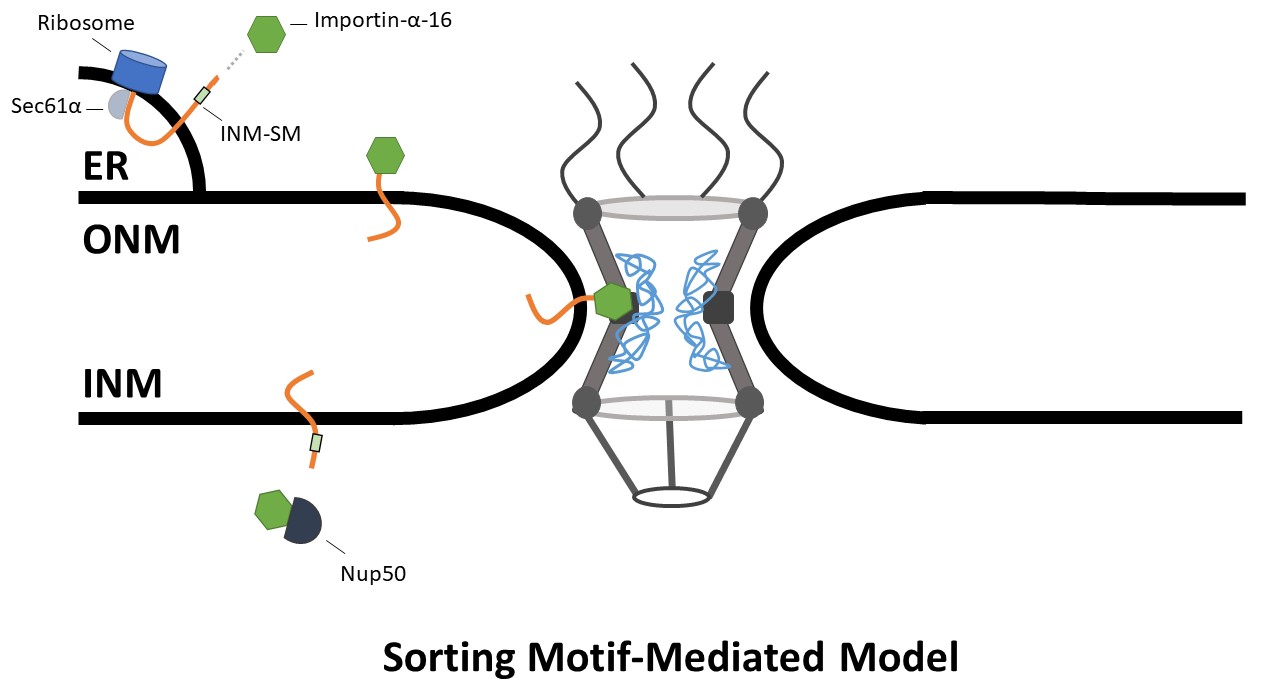
Figure 4. Sorting Motif-Mediated Model. NETs are recognized by Sec61α during translation and are inserted into the membrane. The INM-SM binds to Importin-α16 which then transports the NETs to the INM. Importin-α-16 is then stimulated by Nup50 to release the INM-SM.
2.3. CLASSICAL APPROACHES TO STUDY S







