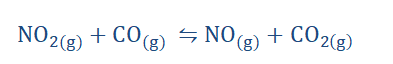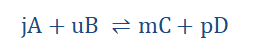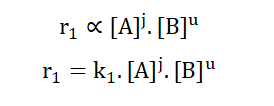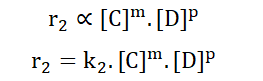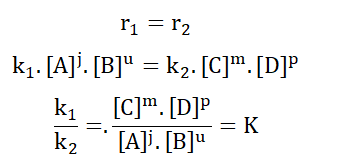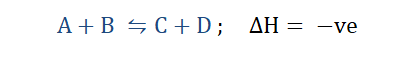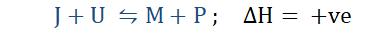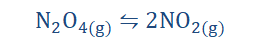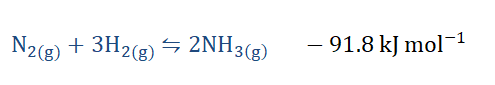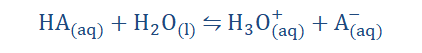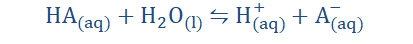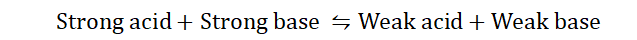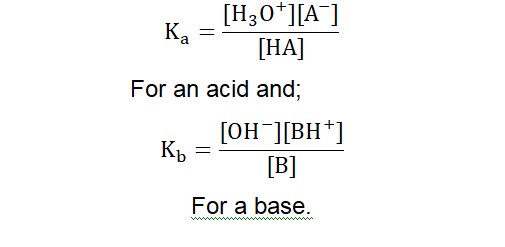CHAPTER 7: EQUILIBRUM
If we were to summarize the aim of all that exists based on what we’ve learnt so far, we would roughly do so in only a word-balance. Balance seems to be nature’s theme.
Everything that occurs, every reaction, the very elementary particles themselves seek some sort of stability. Even in a philosophical sense the essence of life lies in achieving balance. But we are scientists so let’s stick to science. Even when systems seem to be in chaos it is all on account of attempts to attain stability. It almost seems paradoxical but we have seen from the second thermodynamic law that this is so.
In chemical reactions the story is no different. The utility we obtain from products appears to be just a by-product of the real reason for the reaction. That reason is balance or more appropriately called chemical equilibrium .
“Chemical equilibrium in a reversible reaction occurs
when reactant and product concentrations do not
alter with time, and the rate of the forward reaction is
equal to the rate of the backward reaction.”
Equilibrium is of two major types. These are static equilibrium and dynamic equilibrium.
Two other types of equilibrium are homogenous and heterogeneous equilibrium;
dependent on the phases of the reacting substances and their products. When the
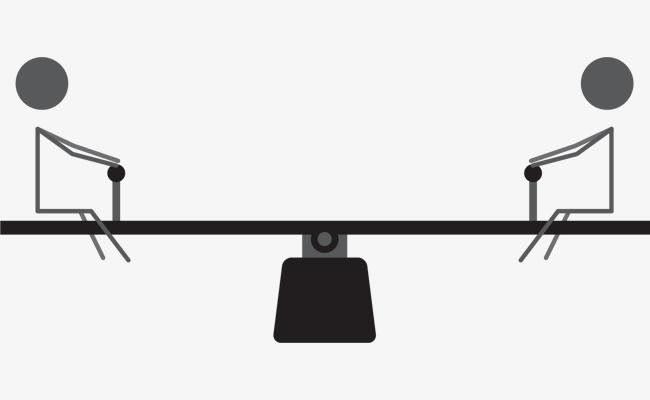
reactants and products are in the same phase the equilibrium is homogenous while in different phases it is heterogeneous. Our main focus here however is on static and dynamic equilibrium. In static equilibrium stationary balance is attained. Both sides of the system are balanced and remain so. An example of this sort of equilibrium can be found in a see-saw. If two children of roughly the same weight sit at equal distance from each other on a see-saw they can be said to attain static or stationary equilibrium. In the second type of equilibrium however, the entities of the system are in constant motion.
Consider two men juggling for instance. As they juggle, each man throws a number of bottles to the other man and the other man does the same. When the number of bottles passed on to each other reaches a constant value, each man receives and passes
176

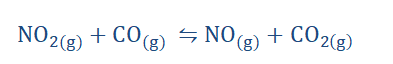
bottles at the same rate. In this situation a dynamic equilibrium can be said to be attained.
Figure 7-1 (a & b) A balanced see-saw and passing in bottle juggling illustrate the two types of equilibrium.
Chemical reactions exhibit dynamic equilibrium. They can also only occur in closed systems. This is because as reactants combine to form products, the products form the reactants as well. If you are keen am certain you recognized this to be a reversible reaction. A closed system is one that is unaffected by external forces or changes. In reactions the closed system is usually in the form of a sealed vessel where nothing can get out and nothing can get in. In that case when reactants form products, the products recombine to form the reactants. This process continues until the rate at which the reactants combine to form the products becomes equal to the rate at which the products recombine to form the reactants. When this point is reached we can say that the
reaction has reached a dynamic equilibrium. For instance let us consider the following reaction;
The forward reaction consists of the reaction between the reactants while the backward reaction concerns the combination of the products to reform the reactants. If this reaction occurs in a closed system equilibrium would be attained. However when it is conducted in an open system such as when the lid of the vessel is removed, the
reaction takes place in only one direction as the CO2 and NO being gasses would
escape into the atmosphere and so would not be able to recombine to form CO and
177
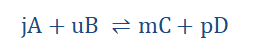
NO2. For this reason having the reaction take place in a closed system is crucial which in the case of this example is a tube. At the beginning, we find that the reddish-brown NO2 and CO react to form the products NO and CO2 which are both colorless gases.
With some time however we begin to notice that the CO2 and NO recombine to form the reactants because the reddish-brown color of NO2 returns to the tube. When the
concentrations of reactants and products become equal we can then say that it is in dynamic equilibrium.
Equilibrium Constant (K) and the law of mass action
We would not be good chemists if we did not find a way to quantitatively measure the equilibrium that accompanies chemical reactions. This measure is known as the
equilibrium constant and is denoted by K. In order to do this we would have to star in
‘the law of mass action’ featuring Guldberg and Waage.
I could not help myself because the names Gulberg and Waage in association with the terms law of mass action just simply sounded like a blockbuster movie. Let me rephrase that a bit.
The law of mass action was explored by two scientists; Cato M. Gulberg and Peter
Waage between the years of 1864 and 1879. Their aim was to understand the
behaviors exhibited by substances and solutions in dynamic equilibrium. They came to the conclusion that concentration was a highly dependent factor on how equilibrium was arrived at by solutions. This concentration is what they had termed ‘mass action’.
“The law of mass action states that under conditions
of constant temperature, the rate of a reaction is
dependent on the active masses (concentration) of
the reactants.”
For a reaction;
A and B are the concentrations of reactants A and B respectively while C and D are the concentrations of the corresponding products. j, u, m and p are amounts in moles. The forward reaction has a rate of r1 while the backward reaction has a rate of r2.
Based on the law of mass action; for the forward reaction;
178
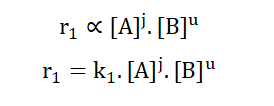
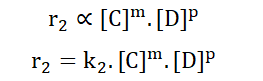
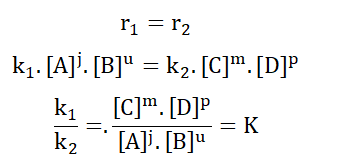
The same applies for the backward reaction;
K is known as the velocity constant. It describes the relative speed of particle collision for each half of the reaction i.e. for the forward and backward reactions independently.
In dynamic equilibrium, the rate of the forward reaction becomes equal to the rate of the backward reaction.
This final K is the equilibrium constant of the entire reaction at a particular temperature since the constant alters with a change in temperature. It helps to show the relationship between reactants and products in a state of equilibrium.
Le Chatelier’s principle
I have a cousin who as a kid was obsessed with having things his way. Indeed as
children we all were intransigent about how we wanted things but he took it to a whole new level. If you placed an object in a different location from where he put it he had the habit of throwing tantrums and just returning it back there. You can imagine how
Christmas dinners were with him.
Just like my cousin, chemical reactions can be terribly ‘stubborn’ but we can’t blame them for being so. Imagine if someone asked you to stand under the mid day sun but you knew that you would be better under a shade. I am quite sure you’ll pick the shade over the scorching sun any day. Chemical reactions do something similar. Whenever a reaction approaches or is at equilibrium and we change any of its conditions, it changes so as to cancel the effect of that change and assume a state of equilibrium. This is the idea behind the principle that was put forth by Henry Louis Le Chatelier in 1884.
179
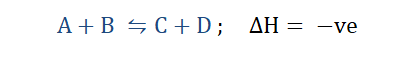
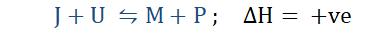
“Le Chatelier’s principle states that for a system in
equilibrium, whenever any of its conditions are
altered its equilibrium shifts so as to annul the effect
of that change.”
We would now examine how the different possible alterations such as changes in
temperature, concentration, pressure and catalyst affect the equilibrium of a system and in so doing we would note how Le Chatelier’s principle may be applied.
Effect of change in temperature on equilibrium constant
When a chemical system attains equilibrium and the temperature of the system is
increased, its response to that change depends on what sort of reaction it is,i.e. whether it is an exothermic or endothermic reaction.
For an exothermic reaction;
When the heat of the system is increased, the system accommodates this change by
releasing the excess heat into the surroundings. On doing so it favors the backward reaction meaning it favors the formation of the reactants. By favoring the backward reaction the equilibrium position shifts to the left and the equilibrium constant K
decreases.
In the event that the temperature of the system is lowered, the equilibrium position shifts to the right and the forward reaction is favored. This means that product formation is favored and the equilibrium constant K increases.
As for an endothermic reaction;
On increasing the heat of the system the equilibrium position shifts to the right, favoring product formation, hence the forward reaction. This also means that K increases. When the temperature falls, the equilibrium position shifts to the left, the backward reaction is favored meaning that reactant formation is favored and K decreases.
Let’s put this in a table so as to clear away any residual confusion.
Temperature of system
Endothermic reaction
Exothermic reaction
180
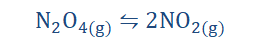
1. Increase in
Equilibrium position
Equilibrium position
temperature
shifts to the right.
shifts to the left.
Favors the forward
Favors the backward
reaction, i.e. product
reaction, i.e. reactant
formation.
formation.
Equilibrium constant
Equilibrium constant
(K) increases.
(K) decreases.
2. Decrease in
Equilibrium position
Equilibrium position
temperature
shifts to the left.
shifts to the right.
Favors the backward
Favors the forward
reaction, i.e. reactant
reaction, i.e. product
formation.
formation.
Equilibrium constant
Equilibrium constant
(K) decreases.
(K) increases.
Effect of change in pressure on equilibrium constant
When dealing with pressure we consider gaseous systems. When gaseous reactants
react to form products equilibrium is attained when the number of moles of reactants and the number of moles of products are equal. A change in pressure implies that this balance of moles is tampered with and so there becomes an uneven distribution in the number of moles of each side. This means that the number of moles on one side
exceeds the other. There is therefore uneven pressure. The system tries to fix this in accordance with Le Chatelier’s principle by favoring the opposite of what external constraint has been applied. For instance if one were to increase the pressure of a system in equilibrium, the system would fix this by favoring the reaction with a reduction in pressure. The adverse response would be gotten when one tries to reduce the
pressure of the system. Have a look at the equation below.
Looking at the forward reaction we observe that there is an increase in the number of moles as reactants convert to products since 1 mole of N2O4 gives 2 moles of NO2. The number of moles is proportional to the pressure, so that a higher mole number indicates 181
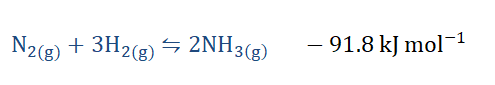
a higher pressure. What this implies is that a reduction in pressure favors the forward reaction while a corresponding increase in pressure favors the backward reaction.
Effect of change in concentration on equilibrium constant
We know that equilibrium is attained when the concentrations of reactants and products are equal. In situations where the concentration of the reactants increase, the system corrects this imbalance by favoring the forward reaction, allowing greater product formation so that equilibrium can be attained.
Effect of catalysts on equilibrium
Catalysts have no effect on the equilibrium of a reaction. This is because they only work by lowering the activation energy of the reaction. They however indirectly affect the attainment of equilibrium by speeding up the reaction and in so doing allow for
equilibrium to be arrived at much faster. In the absence of a catalyst, equilibrium would still be attained but at a much slower rate.
Examples of equilibrium in chemical reactions
1) The Haber-Bosch process: This is an industrial process used in the
manufacture of ammonia for large scale purposes. It was discovered by our
friend Fritz Haber (remember him?) and Carl Bosch when they had found a more
man-made method of nitrogen fixation by converting atmospheric nitrogen into
ammonia due to its reaction with hydrogen. This reaction is an exothermic one
and in a closed system under the right conditions, it has been noted that the
ammonia formed reverts to the substances from which it was made.
If we increase the temperature of the reaction, being an exothermic one it would
adjust to annul that change by favoring the backward reaction (hence favoring
the formation of N2 and H2) thereby shifting the equilibrium position to the left and decreasing the equilibrium constant. However if we lower the temperature more
NH3 would be formed (favoring the forward reaction), the equilibrium position
would shift to the right and the equilibrium constant would increase.
182
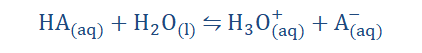
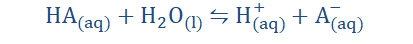
What would happen if we increase the pressure? We can see that on the
reactant side we have a total of 4 moles of the reactants while on the product
side there are 2 moles of the product. Increasing the pressure would therefore
mean that the forward reaction would be favored leading to greater product
formation. Decreasing the pressure would do the opposite of that, favoring the
backward reaction and increasing the amount of reactants formed.
As for concentration, an increase in the amount of reactants would always favor
the forward reaction leading to the production of more NH3.
Finally, catalysts such as iron (Fe) are used to speed up the reaction, and hence make the reaction arrive at equilibrium much faster.
2) Acid-Base equilibrium:
Acids appear to only exhibit acidic properties when they are dissolved in water.
This is because on dissolution the hydrogen ions dissociate and the acidic
substance then exhibits those characteristics known to be displayed by acids.
On dissolution, the hydrogen ions become oxonium or hydronium ions (H3O+)
which we have already explored.
In most equations however the H+ notation is preferred to allow for ease in
analysis and for convenience.
Where A = Acid
Several other substances that are non acidic on contact with water do not display acidic properties but those that are acidic do such as HCl and nitrate. The funny thing is that when these substances are not dissolved in water they have none of
those unique acidic characters. They do not turn blue litmus red or produce
hydrogen when they react with metals. Imagine how you act in different
situations. When you are in the presence of certain people such as your perhaps;
strict parents (if they are) you may not be as ecstatic as when you are in the
presence of your peers. Acidic substances in the same way react differently.
Their acidic properties only show when they dissolve in water.
As a side note, I feel it a duty to warn that you should never on any condition
pour water into acid, but pour the acid into the water instead. This is because the reaction between acids and water is very exothermic and very dangerous as it
183
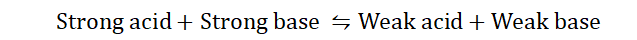
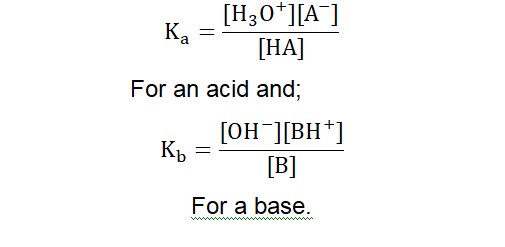
could splash and give you a new face that won’t be as pretty as your present
face. Do not even attempt the acid and water mix unless you are instructed to do
so and under supervision.
In an acid-base reaction, a solution of water and hydronium is known as an acid-
base conjugate pair. This conjugate pair is responsible for the formation of weak acids and bases in solution. Usually in acid-base equilibrium;
The side of the equation with the weaker acid and base is normally favored,
hence favoring the backward reaction, leading to the formation of more reactants.
The equilibrium constants for acid-base reactions can be given by the equations:
184
SUMMARY
Chemical equilibrium in a reversible reaction occurs when reactant and product concentrations do not alter with time, and the rate of the forward reaction is equal to the rate of the backward reaction.
The law of mass action states that under conditions of constant temperature, the rate of a reaction is dependent on the active masses (concentration) of the
reactants.
Le Chatelier’s principle states that for a system in equilibrium, whenever any of its conditions are altered its equilibrium shifts so as to annul the effect of that change.
The equilibrium constant K can be affected by the effect of changes in
temperature, pressure, concentration and the effect of a catalyst.
The Haber-Bosch and acid-base reactions are two instances of equilibrium in
chemical reactions.
185
MNEMONICS
Did you find anything that made you feel the need for a mnemonic today?
The factors that affect the equilibrium constant in chemical reactions:
P
C
C
T
P– Pressure; as in “Change in pressure. ”
C- Concentration; as in “Change in concentration. ”
C- Catalyst; as in“Effect of catalysts. ”
T – Temperature; as in “Change in temperature. ”
‘People Crave Cutting Tires.’ Or ‘People Cut Cakes Together.’ (Some people…)
186
REVISION QUESTIONS
1. Differentiate between reversible and irreversible reactions.
2. State the law of mass action
3. State Le Chatelier’s Principle.
4. State and explain the factors that affect the equilibrium constant K of a recation.
5. When can one say the equilibrium of a reaction has been reached?
6. State and explain two instances of equilibrium in chemical reactions.
7. Differentiate between reaction types that absorb more energy from the
environment and those that lose more energy to the environment in terms of
increase in temperature and decrease in temperature with regards to equilibrium constants.
187