The bigger, the better
The DUBINA EVO rechargeable battery combines many properties. It has a large capacity, charges quickly, weighs a little and is small in size. The car uses a battery with an additional battery to prevent the main battery from being discharged during long-term parking due to parasitic currents in electronic devices. The main battery with a voltage of 400 V has a capacity of 50 kW and an additional voltage of 12 V and a capacity of 1 kW. The main battery consists of 16 modules in each module with 8 batteries. There are 125 batteries in total in the main battery, connected in series. The voltage of one battery is 3.2 V. The capacity of one battery is 125 Ah. The size of one battery is 320x420x12 mm. The battery has dimensions 1780x1300x100 mm. The battery is located under the cockpit floor. Thus, the center of mass is on the centerline, between the front and rear axles of the vehicle at the lowest point. This arrangement distributes the load evenly and does not compromise driving stability.
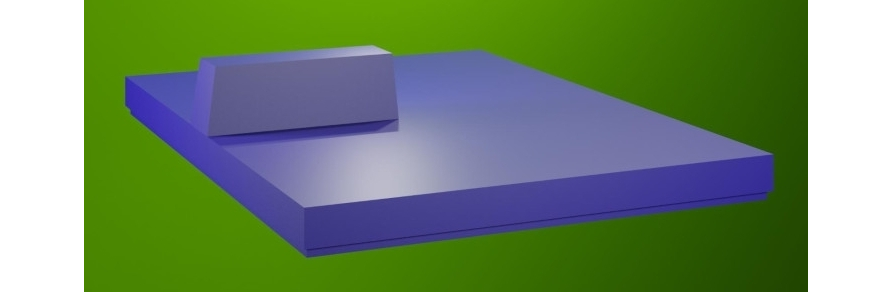
DUBINA EVO battery.
The battery consists of an anode (+) and a cathode (-). The battery capacity C depends on the surface area of the electrode S, the distance between the electrodes d and the conductivity of the electrolyte. The larger the surface area of the electrode S, the larger the battery capacity.
C ~ S,
The smaller the distance between the electrodes d, the greater the battery capacity.
C ~ 1 / d,
The higher the conductivity of the electrolyte, the larger the battery capacity.
С ~ ε,
General formula for battery capacity.
C = εε0 S / d,
Lithium batteries have high energy density and voltage, long service life and low self-discharge, they are commercially available and widely used.
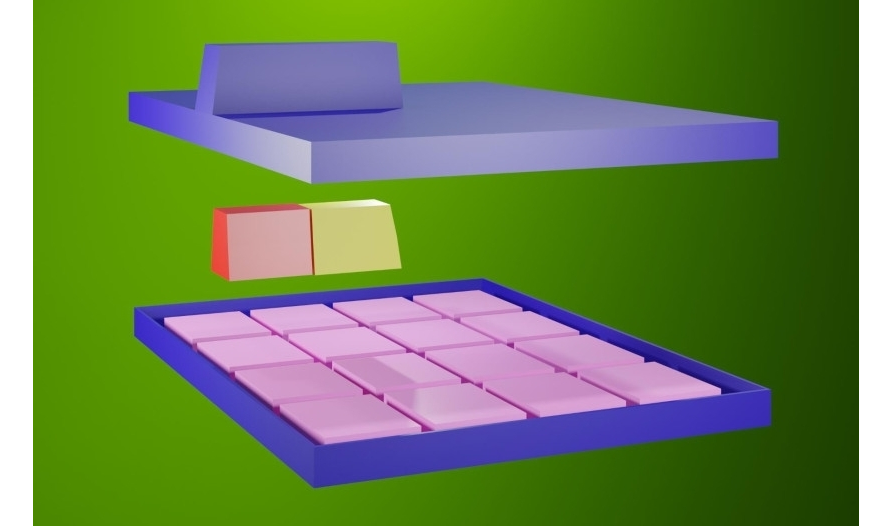
The main parts of the battery.
The cathode (-) of the battery is made of an aluminum current collector coated with LiFePO4. Aluminum is used as a cathode current collector because aluminum has high electrical conductivity, light weight and low cost compared to other materials. Among the olivine structure compounds, LiFePO4 has a high voltage of 3.5 V and a high bulk density of 3.6 g/cm3 compared to lithium, has a theoretical capacity of 170 mAh/g, and exhibits excellent high temperature stability compared to cobalt (Co), and uses cheap Fe, which makes it highly suitable as an active cathode material for lithium batteries. Lithium Iron Phosphate (LiFePO4) olivine type is in the form of secondary particles, in which the primary particles are aggregated and have high porosity, thus providing excellent electrical conductivity and high density, which are the advantages of smaller primary particles, and high process efficiency, which is the advantage of secondary particles.
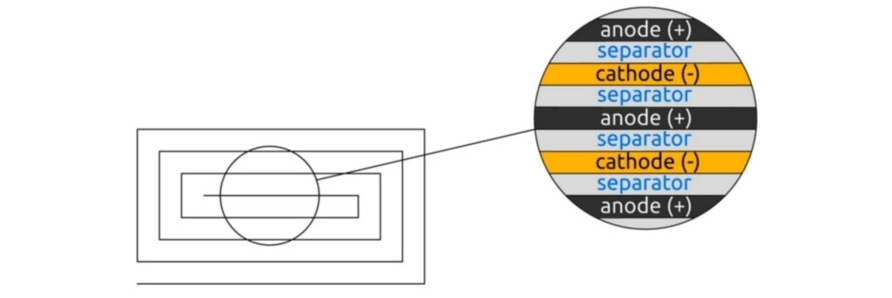
Cross-sectional diagram of a battery accumulator.
The use of lithium iron phosphate of the olivine type in the form of secondary particles can reduce the amount of binder and solvent used to make the electrode and shorten the mixing and drying times. A suspension prepared by mixing the cathode mixture is applied to a current collector 15 μm thick. Cathodic the suspension consists of LiFePO4, a binder, a conductive additive and a solvent. The polyvinylidene binder is a component that facilitates binding of the active material to the conductive material and the collector. The binder is added in an amount of 3% of the total weight of the compound, including the active material of the anode. The conductive material graphite (C) is added in an amount of 3% by weight of the entire cathode. The anode (+) of the battery is made of 100 μm thick graphite foil. The spacer is located between the cathode and anode. A thin insulating film with high ionic permeability and mechanical strength is used as a separator. The separator has a pore diameter of 0.05 μm and a thickness of 10 μm. Non-woven polypropylene is used as a separator.
The lithium content in the upper continental crust is 21 g/t, in seawater — 0.17 mg / l. Lithium deposits are known in Chile, Bolivia, Brazil, Australia, USA, Argentina, Congo, China.
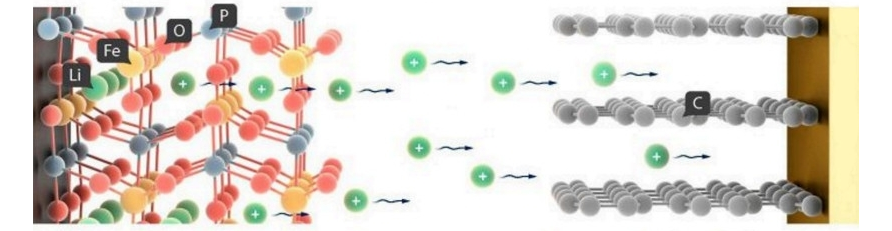
Schematic diagram of the battery.
Battery working mechanism
Charging
Cathode (-) LiFePO4 = Li + FePO4
Anode (+) 6C + Li = LiC6
Discharge
Cathode (-) Li + FePO4 = LiFePO4
Anode (+) LiC6 = 6C + Li

After installing the battery, a threshold is attached to the car body, covering the side of the battery and the lower part of the body.









