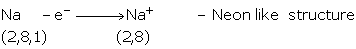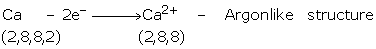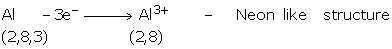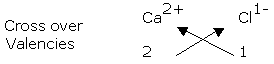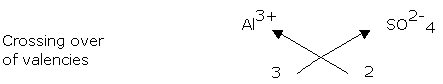3. Predict the occurrence of inert pair effect
4. State and predict the general behaviour of metals, non-metals, and the metal-
loids
5. Predict and explain the trend in the chemical and physical properties of s-block
elements
6. Predict and explain the trend in the chemical and physical properties of p-
block elements
7. Explain, and appreciate the anomalous behaviour of the first elements of a
group
8. State and give example of diagonal relationships in the periodic table
Summary of the learning activity
In the first two Units of this module, we placed and monitored some trends in properties
of the s-, and p-block elements of the periodic table. In this Unit and in furtherance
of the above discussed trends, we monitor the physical and chemical properties of
the s-, and p-block elements. Calculation of the common oxidation state, describing
inert pair effects, and explaining the anomalous behaviour some elements will fol-
low. Diagonal relationship is one subject that most students find fascinating, it will
be considered before tackling relevant worked examples and exercises at the end of
the Unit.
List of Required Readings
1. John C. Kotz and Paul Trichel, Jr . Chemistry & Chemical reactivity, 3rd edi-
tion. Saunders college publishing, NewYork, USA. (1996).
2. Alan G. Sharpe; Inorganic Chemistry, 3rd Edition. Longman Singapore
Publisher (1992).
3. Catherine E. Housecroft and Alan G. Sharpe; Inorganic Chemistry. Prentice-
Hall International, USA. (2000).
4. J. D. Lee, Concise Inorganic Chemistry, 4th edition. Chapman & Hall, New
York. USA. (1993).

African Virtual University
5. Thomas R. Gilbert, Rein V. Kirss, and Geoffrey Davies; Chemistry, The
science in context. W.W. Norton and company NY, USA.(2004).
6. William L. Jolly, Modern inorganic Chemistry 2nd Ed. McGraw-Hill. Inc.
New York, USA (1991).
List of relevant useful links
http://chemistry.about.com/od/elementgroups/a/metals.htm
Outlines what properties distinguishes metals from the other elements.
.http://www.docbrown.info/page01/ElCpdMix/EleCmdMix3.htm
Valency of elements and compounds formation.
http://chemistry.about.com/od/elementgroups/a/alkalimetals.htm
Gives properties for alkali metals
http://www.infoplease.com/ce6/sci/A0859586.htm
Properties of metals
http://chemistry.about.com/od/elementgroups/a/alkalineearths.htm
About Alkali earth metals
http://nobel.scas.bcit.ca/chem0010/unit4/4.3.2_property_nonmetals.htm
Properties of non-metals
http://en.wikipedia.org/wiki/Chalcogen
For chalcogens and their details.
http://www.chemsoc.org/viselements/pages/data/intro_groupvii_data.html
For halogens and their properties.
http://chemistry.about.com/od/elementgroups/a/noblegases.htm
For noble gases
http://www.google.de/search?client=firefox-a&rls=org.mozilla%3Aen-US%3A
official&channel=s&hl=de&q=chemical+properties+of+group+13+element
s&meta=&btnG=Google-Suche (pdf version)
http://www.chemsoc.org/viselements/pages/data/intro_groupiii_data.
Data on general properties of elements of group 13.

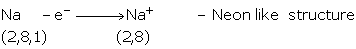
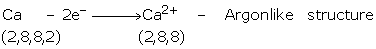
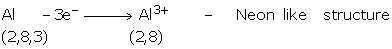
African Virtual University
List of relevant MULTIMEDIA resources
- Computer with internet connecting facility to access relevant links and free
source resourses.
- Multi-media resourses such as CD players, VCD etc.
- CD-ROM for this module for compulsory reading and demonstrations.
Learning activities
Valancy and formulae of compounds
Group IA, IIA and IIIA consists of metals of normal elements. They have 1, 2, or 3
valence electrons, which they can easily donate to acquire the configuration of nearest
noble gas. They become positively charged i.e., cations.
Covalent compounds are formed by the sharing of electrons by atoms of non-metallic
elements for example between groups IVA and VIIA. The elements of these groups
react with one another by sharing election pairs. Thus, the valency of an element in
a covalent molecule is equal to the number of electron pairs shared by an atom of
the element. Normally metals donate electrons from their valence shell so as to form
positively charged ions such that the charge on the ion is equal to its electropositive
valency.
formula of Ionic Compounds
A chemical compound is always electrically neutral.The positive and negative va-
lencies of the ions/radicals in a compound are equal and balanced.

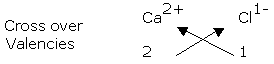
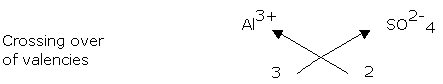
African Virtual University
Steps
1) Write the symbol of the ions of the compound.
2) Cation (positive ion) is written on the left hand side and the anion (negative ion)
is written on the right hand side.
3) Put the valency number of the radical or ion below the symbol of the element.
Alternatively cross the positive and negative charges on ions to give subs-
cripts.
Example: Calcium Chloride Ca2+ Cl1- Valencies 2 1
Formula CaCl .
2
Ensure that the charges are balanced. 1(Ca2+) = +2; 2(Cl-) = -2
4) Radicals need to be enclosed within brackets if their number exceeds one.
Example: Aluminium Sulphate Al3+ SO 2- Valency 3 2
4
2(Al3+) = + 6; 3(SO 2-) = -6 Al (SO ) .
4
2
4 3



African Virtual University
for molecular compounds.
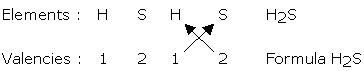
Formula of Molecular Compounds
Steps
1) Write the symbols of the elements which form the compound.
2) Below the symbol of each element write down its valency.
3) Cross over the valencies of the atoms.
note: covalent compounds are formed by the combination between two different non-me-
tals. While writing the formula the less electronegative nonmetal element is written on the
left hand side, whereas, the more electronegative non-metal is written on the right hand side.
Example: HCl
naming of Simple Compounds
Compounds formed by the combination of two different elements are called binary
compounds. Example: CO, CCl , H S, NaCl, NH , HBr. Binary compounds may be
4
2
3
ionic or molecular.
Rules for naming a chemical compound.
Rule 1 The name of the element that occurs first in the compound is written first
without any change in its spelling. In naming MgO, the first part of the formula is
magnesium. If the metal has a variable valency the Roman numeral is included.
For example: PbO would be named as Lead (II) oxide. The name of the second ele-
ment in the formula is written last and is modified to end in ide. For example: MgO
is named Magnesium oxide.
Rule 2 When two nonmetals combine covalently there is a strong chance that more
than one binary compound will be formed. Example: when nitrogen and oxygen
combine depending on the experimental conditions it can form any of the following
compounds N O, NO, N O , NO , N O , N O . In such cases the proportion of the
2
2
3
2
2
4
2
5
various elements are given by the Greek prefix like mono, di, tri, tetra, penta etc.
N O - Di-nitrogen pentoxide NO - Nitrogen dioxide
2
5
2
Rule 3 Compounds containing three elements (ternary compounds), one of which is
oxygen are named with suffix ate at the end, provided there is only one such compound.
If there are two compounds, the one with more oxygen is named with the suffix ate

African Virtual University
and the one with less oxygen is named with -ite ending. Examples: i) a) NaNO 3
- Sodium nitrate b) NaNO - Sodium nitrite ii) a) CaSO - Calcium sulphate b)
2
4
CaSO - Calcium sulphite iii) a) KClO - Potassium chlorate b) KClO - Potassium
3
3
2
chlorite iv) a) Ca (PO ) - Calcium phosphate b) Ca (PO ) - Calcium Phosphite
3
4 2
3
3 2
Rule 4 If in a compound, oxygen is less than the oxygen present in a compound
ending with ite then, it is given the prefix hypo- in the beginning and if oxygen
present in a compound ending with ate is more then it is given the prefix per- in the
beginning. Example: KClO - potassium hypochlorite KClO - potassium chlorite
2
KClO - potassium per chlorate KClO - potassium chlorate
4
3
Charateristics of metals and non-metals
metals
Metals fall into groups in the periodic table determined by similar arrangements of
their orbital electrons and a consequent similarity in chemical properties.
Summary of Common Properties of metals are:
1. shiny metallic luster