Natural Polyphenols and Its Potential in … Sasidharan et al.
grow in the secondary site. In addition,
tions (atherosclerotic diseases affecting
oxidative stress is involved in continuous
arteries that supply heart, brain and lower
angiogenesis through its role in endotheli-
extremities) (Greene et al. , 1992; Rosen
al progenitor activation, release of VEGF
et al. , 2001) and onset of diabetes (Kaya-
and angiopoietin and recruitment of peri-
ma et al. , 2015). Vincent and colleagues
vascular cells.
has demonstrated that ROS production
and neuron injury are activated within 1-2
6. oxidative stress (os) in diabetes pa-
hours of hyperglycaemic insult. Majority
thology
of the patients with impaired glucose tol-
erance have significant peripheral neu-
A growing body of evidence suggest
ropathy, suggesting that ROS induced by
that increased oxidative stress and deficit
hyperglycaemia is critical to cause major
in antioxidant defense mechanism are
diabetes complications (Vincent et al. ,
central players in pathogenesis of diabetes
2002).
complications, in particular β-cell dys-
Metabolic abnormalities such as
function and failure (Folli et al. , 2011).
hyperglycaemia,
hyperlipidaemia,
in-
Under physiological condition, reactive
creased free fatty acids, insulin resistance
oxygen species (ROS) serve as second
and hyperinsulinaemia, each of which
messenger regulates signal transduction
was noted to induce oxidative stress in
and gene expression. Oxidative stress de-
endothelial cells of the blood vessels and
velops from imbalance in redox homeo-
myocardium. In addition, genetic suscep-
stasis (overproduction of mitochondrial
tibility of an individual and presence of
reactive oxygen species (ROS) that ex-
accelerating factors (e. g. hypertension
ceeds the level of antioxidants) leads to
and dyslipidaemia) also contribute to de-
aberrant β-cell function and apoptosis.
velopment of diabetes complications
ROS are heterogenous molecules com-
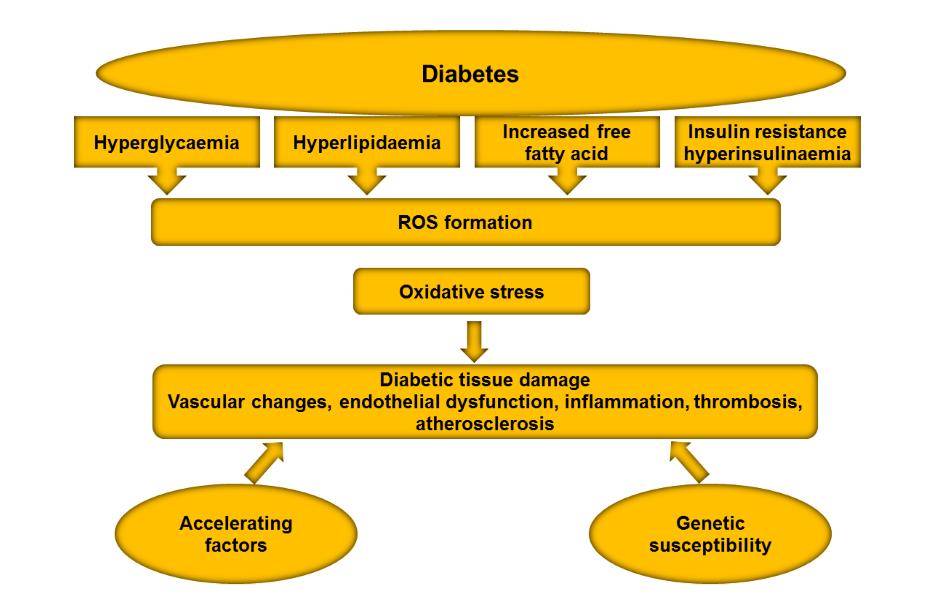
(general features of chronic hyperglycae-
prises of free radicals, such as nitric oxide
mia-induced tissue damage are depicted
(NO. ), superoxide (O. -
2 ), hydroxyl radical
in Figure 3). Several large scale perspec-
(OH. ), non-radicals such as hydrogen per-
tive studies, such as the (Diabetes Control
oxide (H2O2), anions such as superoxide
and Complication Trial DCCT/EDIC
(O -
2 ) and peroxynitrite (ONOOK) (Chang
(The Diabetes Control and Complications
et al. , 1993; Pieper et al. , 1997; Lenzen,
Trial Research Group, 1993), UK pro-
2008; Newsholme et al. , 2012; Cao and
spective Diabetes Study (UKPDS) (UK
Kaufman, 2014; Keane et al. , 2015).
Prospective Diabetes Study (UKPDS)
Sources of free radicals production in-
Group, 1998), and Steno 2 Study have
clude the mitochondrial electron transport
concluded that chronic hyperglycaemia as
system, NADPH oxidases, xanthine oxi-
a key risk factor underlying diabetes pa-
dase (primary source in cardiomyocytes),
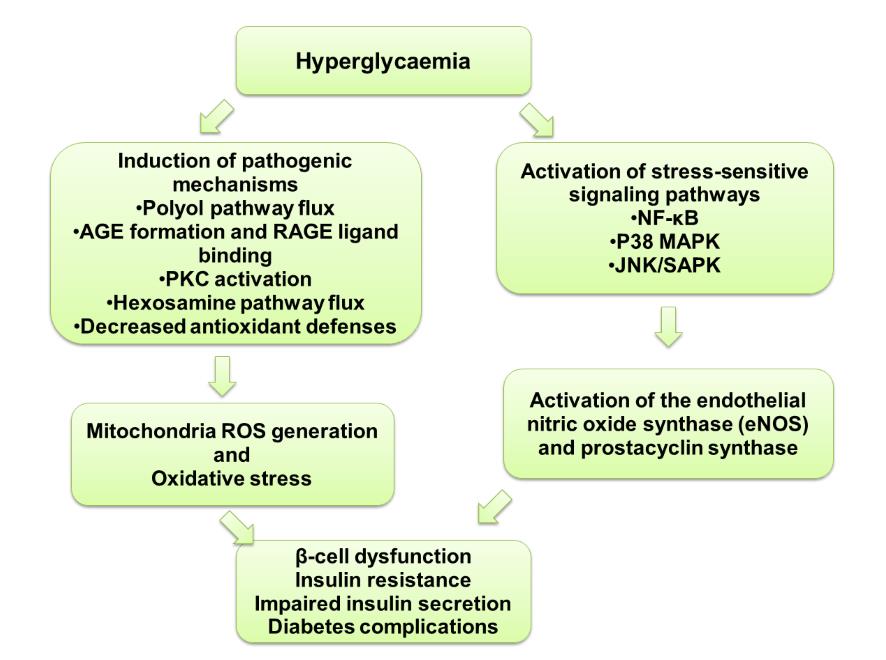
thology (Gaede et al. , 2008). Hypergly-
uncoupled nitric oxide synthase (NOS)
caemia is known to trigger oxidative
and arachidonic acid (primary source in
stress through FIVE major molecular
vascular cells) pathway. Mitochondria are
mechanisms (Figure 4 depicts the mecha-
major source of free radicals production
nism underlying oxidative stress and dia-
in cells. ROS was noted as a key upstream
betes pathology): (1) Activation of Polyol
signaling event mediates downstream
pathway (2) Increased intracellular ad-
metabolic pathways, leading to loss of
vanced glycation end products (AGEs)
cellular biological function and ultimately
pathway activity and receptor expression
cell death (Brownlee, 2005). Ample evi-
for AGEs (RAGE) (3) Activation of Pro-
dence indicate that ROS damage plays a
tein Kinase C isoforms (PKC) (4) In-
major role in pathogenesis of micro- (dia-
creased Hexosamine pathway flux (5)
betic retinopathy, nephropathy, and neu-
Decreased antioxidant defenses (Sima et
ropathy) and cardiovascular complica-
al. , 1990; Engerman et al. , 1994; Brown-
ISBN: 978-967-14475-3-6; eISBN: 978-967-14475-2-9 148
Biotech Sustainability (2017)
Natural Polyphenols and Its Potential in … Sasidharan et al.
Figure 3: General features of chronic hyperglycaemia-induced diabetic tissue damage
(Giacco and Brownlee, 2010).
Figure 4: Mechanisms underlying hyperglycaemia-induced pathophysiology of diabetes
via the generation of ROS and activation of stress-sensitive signaling pathways. Each
mechanism is discussed in the text (Vincent et al. , 2004).
ISBN: 978-967-14475-3-6; eISBN: 978-967-14475-2-9 149
Biotech Sustainability (2017)
Natural Polyphenols and Its Potential in … Sasidharan et al.
lee, 1995; Lee et al. , 1995; Ganz and
1988; Li et al. , 1996). These results in
Seftel, 2000; Brownlee, 2005). The effect
auto-oxidation of glucose to glyoxals, de-
of oxidative stress damage is aggravated
composition of the Amadori product (glu-
by inactivation of anti-atherosclerotic en-
cose-derived 1-amino 1-deoxyfructose
zymes (endothelial nitric oxide synthase
lysine adducts, to 3-deoxyglucosone, and
(eNOS) and prostacyclin synthase. In ad-
fragmentation
of
glyceraldehyde-3-
dition, oxidative stress also activates
phosphate and dihydroxyacetone phos-
stress-sensitive signaling pathways, such
phate to methylglyoxal (Brownlee, 2001).
as nuclear redox sensitive transcription
In addition, increased AGEs production
factor (NF-κB), p38 MAPK, and NH2-
promotes the binding of AGEs to its re-
terminal Jun kinases/stress-activated pro-
ceptors (RAGE). Binding of AGEs to
tein kinases (JNK/SAPK) leads to both
RAGE induces overproduction of ROS
insulin resistance and impaired insulin
and activation of NF-kB signaling and
secretion (Folli et al. , 2011; Brownlee,
upregulation of intracellular adhesion
2001).
molecule-1(ICAM-1), vascular adhesion
cell molecule-1 (VCAM-1), monocyte
7. Molecular mechanisms of hypergly-
chemotactic protein-1 (MCP-1), PAI-1,
caemia-induced oxidative stress in
tissue factor, and VEGF (Yamagishi et
diabetes
al. , 1997; Bierhaus et al. , 2001).
Previous studies demonstrated that
Hyperglycaemia-induced activation of
PCK activity was increased in the retina,
polyol pathway was the first mechanism
kidney and microvasculature of diabetic
discovered (Gabbay et al. , 1966). This
rats (Craven, P.A. and F.R. DeRubertis,
pathway has been therapeutic target for
1989; Lee et al. , 1989), suggested that the
diabetes neuropathy (Oates and Mylari,
lipolytic pathway and production of di-
1999). Recent human genetic study has
acylglycerol induces PKC activation
implicated polymorphisms of the aldose
(Ishii et al. , 1998). Hyperglycaemia in-
reductase gene associated with increased
creases diacylglycerol synthesis, which is
risk for diabetes complications (Oates and
a critical activating co-factor for PKC
Mylari, 1999). Excess glucose activates
isoforms (Derubertis and Craven, 1994;
polyol pathway. Aldose reductase (de-
Xia et al. , 1994; Koya et al. , 1997; Koya
pendent upon NADPH as co-factor) in-
and King, 1998). PKC activation has been
creases conversion of glucose to polyal-
shown to have diverse effects on gene ex-
cohol sorbitol. Excessive activation of
pression in different cell types. PKC acti-
polyol pathway results in depletion of in-
vation inhibits insulin-stimulated endothe-
tracellular NADPH and GSH which is an
lial Nitric Oxide Synthase (eNOS) ex-
important intracellular antioxidant (Lee
pression in the endothelial cells and de-
and Chung, 1999). Accumulation of sor-
creases nitric oxide production in the
bitol forms cellular osmotic stress (Ste-
smooth muscle cells (Vlassara et al. ,
vens et al. , 1993).
1995). In vascular smooth muscle cells,
Excess glucose induces auto-
PKC activation induces over-expression
oxidation through activation of the AGEs
of fibrinolytic inhibitor, plasminogen ac-
pathway.
tivator inhibitor (PAI-1) and activation of
The AGE precursor damages cells by
NF-kB (Abordo and Thornalley, 1997).
three mechanisms: modification of pro-
PKC enhances accumulation of microvas-
teins involve in gene transcription
cular matrix protein by up-regulation of
(Giardino et al. , 1994; Shinohara et al. ,
transforming growth factor (TGF-β), fi-
1998), modification of extracellular ma-
bronectin and type 4 collagen in both cul-
trix molecules (McLellan et al. , 1994),
ture mesangial cells and glomeruli of dia-
and modification of circulating protein in
betic rats (Doi et al. , 1992). PKC also en-
the blood (e. g. albumin) (Vlassara et al. ,
hances vascular permeability by increas-
ISBN: 978-967-14475-3-6; eISBN: 978-967-14475-2-9 150
Biotech Sustainability (2017)
Natural Polyphenols and Its Potential in … Sasidharan et al.
ing the expression of vascular endothelial
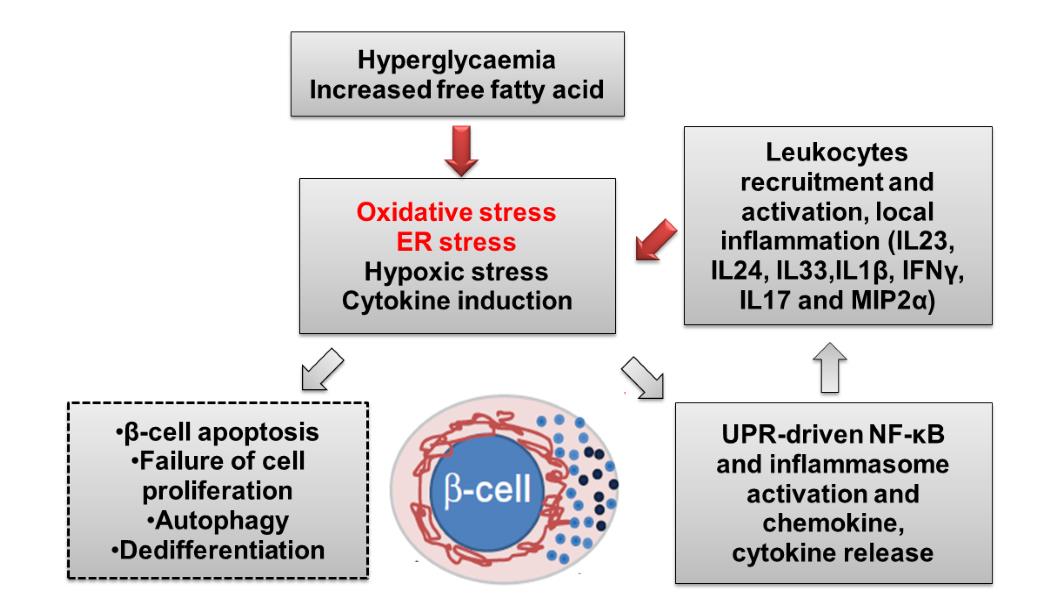
creatic β-cells triggers oxidative stress
growth factor (VEGF) (Skolnik et al. ,
and ER stress, exacerbated by high circu-
1991).
lating glucose and lipids (non-esterified
Lastly, hyperglycaemia causes
fatty acid). Oxidative stress and ER stress
damage to the blood vessel through acti-
induce chemokine production and acti-
vation of hexosamine pathway. The end
vates inflammatory cells in the pancreatic
product of this pathway, uridine diphos-
islet. In turn, the activated inflammatory
phate (UDP)-N-acetyl glucosamine regu-
cells produce cytokines that further exac-
lates gene expression implicated in vascu-
erbate oxidative and ER stress and disrupt
lar complications (such as PAI -, TGF-α,
β-cell secretory pathway function. In ad-
TGF-β1). In addition, activation of hex-
dition, oxidative stress induces unfolded
osamine pathway impairs Insulin Recep-
protein response (UPR) and NF-κB acti-
tor Substrate (IRS)/phosphatidylinositol
vation. In early diabetes (manifested by
3-kinase (PI3-K)/Akt pathway, resulting
chronic ER stress and inflammation), in-
in deregulation of eNOS activity (Bucala
creased proinsulin:insulin ratio impairs
et al. , 1991; Kolm-Litty et al. , 1998).
insulin signaling further aggravates hy-
perglycaemia. Overall, this vicious cycle
8. Oxidative stress and β-cell dysfunc-
leads to β-cell apoptosis and progression
tion in diabetes
to diabetes (summarized schematically in
Figure 5). Several mechanisms have been
Diabetes mellitus (DM) is character-
implicated in β-cell apoptosis (Nakagawa
ized by failure of the pancreatic β-cells to
et al. , 2000; Oyadomari et al. , 2002;
maintain glucose homeostasis. Physiolog-
Puthalakath et al. , 2007; Song et al. ,
ically, the pancreatic β-cells secrete hor-
2008; Mahdi et al. , 2012; Supale et al. ,
mone insulin and regulate glucose home-
2012;
Uruno
et
al. ,
2015).
The
ostasis. Insulin drives glucose uptake in
PERK/ATF4-mediated
activation
of
the liver (reducing hepatic gluconeogene-
CHOP
and
IRE1a/TRAF2/ASK1-
sis both directly and in conjunction with
mediated activation of JNK are important
suppression of glucagon secretion), mus-
molecular mechanisms (reviewed in Papa
cle and fat (Könner, 2007). Because of
FR 2012) (Papa, 2012). A growing body
their high biosynthetic load and require-
of evidence suggests that ER stress induc-
ment for oxygen, pancreatic β-cells are
es autophagy (an important mechanism
very vulnerable to oxidative stress (Len-
for removal of terminally misfolded pro-
zen, 2008; Newsholme et al. , 2012; Cao
tein from the endoplasmic reticulum (ER)
and Kaufman, 2014; Kaneto and Mat-
leads to induction of apoptosis (Wang et
suoka, 2015).
al. , 2014; Li et al. , 2012; Quan et al. ,
Oxidative stress and endoplasmic
2012). Another mechanism involves NF-
reticulum stress (ER) are key pathological
kB and interleukin 1 beta (IL1b) activa-
features in particular type 2 diabetes
tion (reviewed in Hasnain SZ et al. , 2012;
mellitus (T2DM), contribute to pancreatic
Hasnain et al. , 2014).
β-cell dysfunction, inducing inflammation
(immune activation) and β-cell apoptosis.
9. Current therapeutics in diabetes
Previous studies have suggested the oxi-
dative stress is able to suppress insulin
Good glycaemic control is the most effec-
transcription and associated with accumu-
tive mean of mitigating diabetes compli-
lation of β-amyloid in the human pancre-
cations in particular type 1 diabetes
atic islet (Kaneto and Matsuoka, 2015). In
(Greene et al. , 1992; Molitch et al. ,
obesity and early stage of diabetes, nutri-
1993). In general, drug available for dia-
ent overload leads to development of mild
betes work by reducing stress on β-cells
insulin resistance and hyperglycaemia.
biosynthesis pathway. In diabetes pa-
Increased insulin production by the pan-
tients, the use of drug that promotes insul-
ISBN: 978-967-14475-3-6; eISBN: 978-967-14475-2-9 151
Biotech Sustainability (2017)
Natural Polyphenols and Its Potential in … Sasidharan et al.
Figure 5: Schematic representation of the cycle of oxidative and ER stress and its effects
on glucose homeostasis in diabetes (Hasnain et al. , 2015).
-in secretion (such as sulfonylureas) is
10. Biological effects of natural poly-
known to causes loss of β-cell function.
phenols on oxidative stress
Another class of GLP-1 receptor agonist,
which promotes insulin secretion in a glu-
Oxygen is an essential element of life
cose dependent-manner, may also have
used by cells to generate energy in the
long-term damaging effect on β-cell
form of ATP whereby this process occurs
(Hasnain et al. , 2014). Drug that sup-
within the mitochondria (Turrens, 2003).
presses gluconeogenesis (metformin), in-
The process, however, causes the produc-
crease glucose excretion (SGLT-2 inhibi-
tion of free radicals, such as reactive oxy-
tor) or reduces peripheral insulin re-
gen species (ROS) and reactive nitrogen
sistance (thiazolidinediones) or exoge-
species (RNS) due to the cellular redox
nous insulin.
process in the cells (Pham-Huy et al. ,
Given that pronounced oxidative
2008). At low or moderate concentration,
stress mediates major diabetes complica-
these species exert beneficial effects on
tions, antioxidant therapy remains a novel
cellular responses and immune function,
therapeutic approach for diabetes patients.
but when the species exist at higher lev-
Antioxidant drugs target NADPH oxidas-
els, oxidative stress is generated (Young
es are unable to combat high level of oxi-
and Woodside, 2001; Halliwell, 2007;
dative stress (Li et al., 2012). In view that
Pham-Huy et al. , 2008). Oxidative stress
the IL22 receptor is the most highly ex-
refers to the balance between the produc-
pressed in human pancreatic islet cells,
tion of free radicals and antioxidant de-
studies have been identifying IL22 as
fences in a cell (Betteridge, 2000). The
novel antioxidant target in diabetes (Co-
mechanism arises when there is an unfa-
bleigh and Robek, 2013; Kumar et al. ,
vourable balance between the free radical
2013; Rutz et al. , 2013; Hasnain et al. ,
production and antioxidant defences,
2014; Sabat et al. , 2014).
which result in the damage of a broad
range of molecular species, including li-
ISBN: 978-967-14475-3-6; eISBN: 978-967-14475-2-9 152
Biotech Sustainability (2017)
Natural Polyphenols and Its Potential in … Sasidharan et al.
pids, proteins and nucleic acid in the cells
Polyphenols are a large group of
(Rock et al. , 1996; McCord, 2000).
natural antioxidants found mostly in
The concept of oxidative stress
fruits, vegetables, cereals and beverages
was first hypothesised in the 1950s by
(Arts and Hollman, 2005; Pandey and
researchers that investigated the toxic ef-
Rizvi, 2009). There are more than 8,000
fects of ionizing radiation, free radicals,
polyphenolic compounds that have been
and the similar toxic effects of molecular
identified in various plant species, which
oxygen (Gerschman et al. , 1954), as well
arise from a common intermediate, phe-
as its possible contribution to the aging
nylalanine or an immediate precursor, and
process (Harman, 1956). Interest in this
shikimic acid. Polyphenols contain phenol
field of research grew (Hybertson et al. ,
rings in the basic structure. Based on the
2011) when studies reported that the bio-
number of phenol rings and the basis of
logical systems are capable of producing
the structural elements that binds to these
substantial amounts of superoxide free
rings, polyphenols can be classified as
radical, O -
2 through the natural metabolic
phenolic acids, flavonoids, stilbenes and
pathways (McCord & Fridovich, 1968)
lignans (Spencer et al. , 2008; Pandey and
and the activity






